Press release
GMP Testing Service Market 2023-2033: Ensuring Quality and Compliance in a Dynamic Landscape
The global Good Manufacturing Practice (GMP) testing service market is poised for significant growth from 2023 to 2033 as industries continue to prioritize quality assurance, regulatory compliance, and consumer safety. GMP testing services play a pivotal role in ensuring that products meet stringent quality standards and regulatory requirements, fostering trust among consumers and stakeholders. This article provides an in-depth analysis of the GMP testing service market, including its overview, market size and growth, segmentation, regional analysis, drivers and challenges, trends, future outlook, key study points, competitive landscape, and recent developments.The global GMP Testing Service market was valued at US$ 1900 Million in 2022 and is expected to expand at a CAGR of 5.4% from 2022 to 2032. Factors such as the expanding pharmaceutical sector and increased medication and device development are projected to drive market expansion.
Ready to outshine your competitors in the GMP Testing Service Market? Request a sample and gain invaluable insights on competitor analysis today @ https://www.persistencemarketresearch.com/samples/33233
Market Drivers and Challenges: Several factors contribute to the growth of the GMP testing service market. Stringent regulatory requirements, an emphasis on product quality and safety, evolving consumer preferences, and the increasing adoption of advanced testing technologies are key drivers. However, challenges such as the high cost of advanced testing equipment, shortage of skilled personnel, and variations in regulatory standards across regions can impede market growth.
Market Trends: The GMP testing service market is characterized by several notable trends:
Automation and Technology Integration: The integration of advanced technologies such as robotics, artificial intelligence, and machine learning into testing processes enhances efficiency, accuracy, and data analysis.
Rise of Biopharmaceuticals: The growing focus on biopharmaceuticals and biologics necessitates specialized GMP testing services tailored to these complex products.
Outsourcing to Third-Party Laboratories: Many companies are opting to outsource GMP testing services to specialized laboratories, allowing them to focus on core competencies.
Environmental Monitoring: Increased attention to environmental monitoring and contamination control drives the demand for microbiological testing services.
Ready to seize the business opportunity and uncover the true market value in the GMP Testing Service Market? Purchase our premium insight now and unlock your potential @ https://www.persistencemarketresearch.com/checkout/33233
Future Outlook: The future of the GMP testing service market appears promising, with an increasing number of companies recognizing the strategic importance of adhering to stringent quality and compliance standards. As industries continue to expand and innovate, the need for comprehensive GMP testing services will only intensify.
Key Market Study Points: Researchers and analysts studying the GMP testing service market should focus on the following key points:
Regulatory Landscape: A detailed analysis of the evolving regulatory requirements and their impact on the demand for GMP testing services.
Technology Adoption: Exploration of the integration of advanced technologies and automation in GMP testing processes.
Industry-Specific Requirements: Understanding the unique GMP testing needs of different industries and verticals.
Market Penetration: Assessment of the strategies employed by key market players to gain a competitive edge.
Recent Developments: In recent years, the GMP testing service market has witnessed notable developments, including:
Key players expanding their testing capabilities through acquisitions of specialized testing laboratories.
Introduction of innovative testing methodologies, such as rapid microbial detection and next-generation sequencing.
Collaborations between testing service providers and regulatory authorities to streamline compliance processes.
Seeking specific insights on segments, regions, or competitors in the GMP Testing Service Market? Customize your research and request for tailored insights today @ https://www.persistencemarketresearch.com/request-customization/33233
Key Segments in GMP Testing Service Industry Research
By Type
Product Validation Testing
Bioanalytical Services
Packaging & Shelf-Life Testing
Other Services Type
By End User
Pharmaceutical and Biopharmaceutical Companies
Medical Device Companies
By Region
North America
Latin America
Europe
Asia Pacific
Middle East & Africa
Top Market Research Reports:
Wound Sprays Market: https://www.globenewswire.com/en/news-release/2023/02/13/2606548/0/en/Wound-Sprays-Market-is-estimated-to-be-worth-US-728-9-million-by-2032-end-at-a-CAGR-of-4-4-Study-by-Persistence-Market-Research.html
Medicated Soap Market: https://www.globenewswire.com/news-release/2023/02/15/2608746/0/en/Medicated-Soap-Market-to-Cross-US-26-2-billion-in-Revenues-by-the-end-of-2032-says-Persistence-Market-Research.html
About us
Persistence Market Research
Contact Us:
Address - 305 Broadway, 7th Floor, New York City, NY 10007 United States
10007
United States
Phone : 06465687751
Media Contact:
Persistence Market Research
305 Broadway,7th Floor New York City, NY 10007 United States
Call +1-646-568-7751
Call +1 800-961-0353
sales@persistencemarketresearch.com
Persistence market Research comes across as an incomparable provider of market intelligence from the other side of the fence. In other words, Persistence Market Research, with all its pragmatism, perseverance, and prudence, brings the nitty-gritties of market research for the clients, to the service of clients, and abides by the objective of guiding clients in profitable approach.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Testing Service Market 2023-2033: Ensuring Quality and Compliance in a Dynamic Landscape here
News-ID: 3164933 • Views: …
More Releases from Persistence Market Research

Textile Flooring Market Set for Steady Growth as Demand for Sustainable and Styl …
The global textile flooring market is entering a phase of stable expansion, supported by rising construction activity, increasing consumer focus on interior aesthetics, and growing demand for eco-friendly flooring solutions. According to industry estimates, the global textile flooring market size is likely to be valued at US$11.1 billion in 2026 and is projected to reach US$16.5 billion by 2033, expanding at a CAGR of 5.8% between 2026 and 2033. This…

Power System Simulator Market Size to Reach US$ 2.6 Billion by 2033 - Persistenc …
The power system simulator market is gaining strategic importance as global energy systems transition toward digitalization, decentralization, and decarbonization. Power system simulators are advanced software and hardware platforms used by utilities, grid operators, engineering firms, and research institutions to model, analyze, and optimize electrical power networks. These simulators enable real time grid analysis, contingency planning, load flow studies, fault analysis, stability assessment, and operator training. As electricity networks become more…

Yoga and Meditation Products Market Set for Robust Growth, Projected to Reach US …
The global wellness industry is undergoing a major transformation as consumers increasingly prioritize mental health, mindfulness, and preventive self-care. Within this evolving landscape, the yoga and meditation products market has emerged as a fast-growing segment, encompassing everything from yoga mats and apparel to meditation cushions, smart devices, and digital-enabled accessories. According to industry estimates, the global yoga meditation products market is projected to be valued at US$ 8.3 billion in…
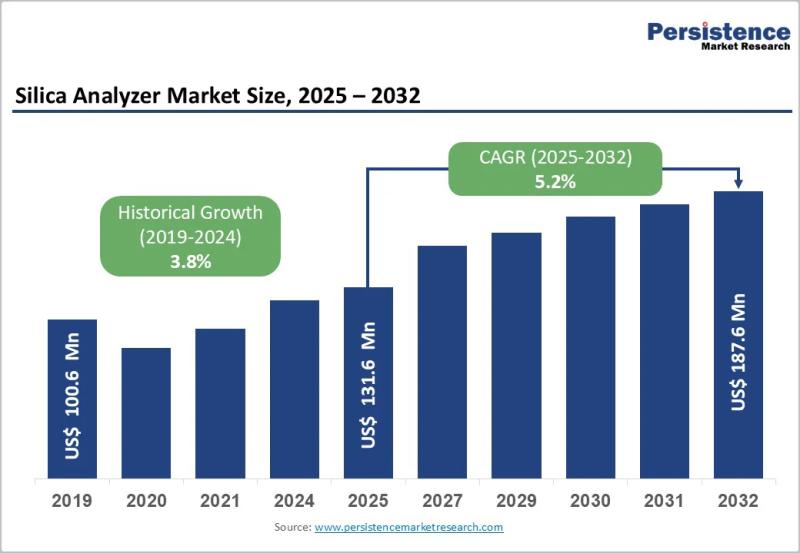
Silica Analyzer Market Size to Reach US$187.6 Million by 2032 - Persistence Mark …
The silica analyzer market plays a critical role in industrial water quality monitoring, particularly in sectors where high purity water is essential for operational efficiency and equipment longevity. Silica analyzers are specialized instruments used to detect and measure silica concentrations in water and steam cycles, preventing scale formation and corrosion in boilers, turbines, and cooling systems. Industries such as power generation, oil and gas, pharmaceuticals, semiconductors, and chemical processing rely…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
