Press release
A New Era in Cerebral Adrenoleukodystrophy Treatment: DelveInsight's Cerebral Adrenoleukodystrophy Clinical Trials Report Highlights a Pathway to Improved Therapies | Minoryx Therapeutics, POXEL SA, Orpheris Inc, Bluebird Bio Inc, Magenta Therapeutics Inc
(Albany, United States) As per DelveInsight's assessment, globally, the Cerebral Adrenoleukodystrophy pipeline constitutes 3+ key companies continuously working towards developing 3+ Cerebral Adrenoleukodystrophy treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analysis DelveInsight.In the Cerebral Adrenoleukodystrophy pipeline report, detailed description of the drug is given which includes mechanism of action of the drug, clinical studies, Cerebral Adrenoleukodystrophy NDA approvals (if any), and product development activities comprising the technology, Cerebral Adrenoleukodystrophy collaborations, licensing, mergers and acquisition, funding, designations and other product related details.
To explore more information on the latest breakthroughs in the Cerebral Adrenoleukodystrophy pipeline treatment landscape of the report, click here @ Cerebral Adrenoleukodystrophy Pipeline- https://www.delveinsight.com/report-store/cerebral-adrenoleukodystrophy-cald-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Key Takeaways from the Cerebral Adrenoleukodystrophy Pipeline Report
• DelveInsight's Cerebral Adrenoleukodystrophy Pipeline analysis depicts a robust space with 3+ active players working to develop 3+ pipeline treatment therapies.
• The leading Cerebral Adrenoleukodystrophy Companies working in the market include Minoryx Therapeutics, POXEL SA, Orpheris Inc, Bluebird Bio Inc, Magenta Therapeutics Inc, SwanBio Therapeutics Inc, and others
• Promising Cerebral Adrenoleukodystrophy Pipeline Therapies in the various stages of development MIN-102, OP-101, MGTA-456, and others.
• On July 2023, Minoryx Therapeutics Inc announced a study of phase 2 clinical trials for MIN-102. An Open-Label, multicenter study in male pediatric patients with cerebral x-linked adrenoleukodystrophy (cald) to assess the effects of MIN-102 treatment on disease progression prior to human stem cell transplant (HSCT).
• On March 2023, BlueBird Bio announced a study of phase 3 clinical trials for Lenti-D. The purpose of this study is to evaluate the efficacy and safety of Lenti-D Drug Product (also known as elivaldogene autotemcel or Skysona, hereafter referred to as eli-cel) after myeloablative conditioning with busulfan and fludarabine in participants with CALD. A participant's blood stem cells will be collected and modified (transduced) using the Lenti-D lentiviral vector encoding human adrenoleukodystrophy protein.
Cerebral Adrenoleukodystrophy Overview
Cerebral adrenoleukodystrophy - or CALD - is a rare and devastating neurologic disease that robs young patients of the chance to live a full life. The disease results in rapid loss of neurological function after the initial onset of symptoms and sadly, nearly half of patients who do not receive treatment die within five years of symptom onset.
To explore more information on the latest breakthroughs in the Cerebral Adrenoleukodystrophy Pipeline treatment landscape of the report, click here @ https://www.delveinsight.com/sample-request/cerebral-adrenoleukodystrophy-cald-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Cerebral Adrenoleukodystrophy Emerging Drugs Profile
• MIN-102: Minoryx Therapeutics
• PXL770: POXEL SA
Cerebral Adrenoleukodystrophy Pipeline Therapeutics Assessment
There are approx. 3+ key companies which are developing the therapies for Cerebral Adrenoleukodystrophy. The Cerebral Adrenoleukodystrophy companies which have their Cerebral Adrenoleukodystrophy drug candidates in the most advanced stage, i.e. phase II/III include, Minoryx Therapeutics.
Request a sample and discover the recent advances in Cerebral Adrenoleukodystrophy Ongoing Clinical Trial Analysis and Medications, click here @ https://www.delveinsight.com/sample-request/cerebral-adrenoleukodystrophy-cald-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Cerebral Adrenoleukodystrophy Drugs and Companies
• MGTA-456: Magenta Therapeutics Inc
• OP-101: Orpheris Inc
• MIN-102: Minoryx Therapeutics S.L.
Cerebral Adrenoleukodystrophy Therapeutics Assessment
• Assessment by Stage and Product Type
• Assessment by Route of Administration
• Assessment by Stage and Route of Administration
• Assessment by Molecule Type
• Assessment by Stage and Molecule Type
Some of the Companies in the Cerebral Adrenoleukodystrophy Therapeutics Market include-
Minoryx Therapeutics, POXEL SA, Orpheris Inc, Bluebird Bio Inc, Magenta Therapeutics Inc, SwanBio Therapeutics Inc, and others.
Dive deep into rich insights for drugs for Cerebral Adrenoleukodystrophy Pipeline, click here for Cerebral Adrenoleukodystrophy Unmet Needs and Analyst Views @ https://www.delveinsight.com/sample-request/cerebral-adrenoleukodystrophy-cald-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Scope of the Cerebral Adrenoleukodystrophy Pipeline Report
• Coverage- Global
• Cerebral Adrenoleukodystrophy Companies- Minoryx Therapeutics, POXEL SA, Orpheris Inc, Bluebird Bio Inc, Magenta Therapeutics Inc, SwanBio Therapeutics Inc, and others.
• Cerebral Adrenoleukodystrophy Pipeline Therapies- MIN-102, OP-101, MGTA-456, and others.
• Cerebral Adrenoleukodystrophy Segmentation: Product Type, Molecule Type, Route of Administration
Got Queries? Find out the related information on Cerebral Adrenoleukodystrophy Mergers and acquisitions, Licensing Activities @ https://www.delveinsight.com/sample-request/cerebral-adrenoleukodystrophy-cald-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=ypr
Table of Content
1. Introduction
2. Executive Summary
3. Cerebral Adrenoleukodystrophy: Overview
4. Pipeline Therapeutics
5. Therapeutic Assessment
6. Cerebral Adrenoleukodystrophy - DelveInsight's Analytical Perspective
7. Late Stage Products (Registered)
8. elivaldogene autotemcel (eli-cel): bluebird bio, Inc.
9. Drug profiles in the detailed report…..
10. Mid Stage Products (Phase II/III)
11. MIN-102: Minoryx Therapeutics
12. Drug profiles in the detailed report…..
13. Early Stage Products (Phase I)
14. Drug name: Company name
15. Drug profiles in the detailed report…..
16. Preclinical and Discovery Stage Products
17. Drug name: Company name
18. Drug profiles in the detailed report…..
19. Inactive Products
20. Cerebral Adrenoleukodystrophy Key Companies
21. Cerebral Adrenoleukodystrophy Key Products
22. Cerebral Adrenoleukodystrophy- Unmet Needs
23. Cerebral Adrenoleukodystrophy- Market Drivers and Barriers
24. Cerebral Adrenoleukodystrophy- Future Perspectives and Conclusion
25. Cerebral Adrenoleukodystrophy Analyst Views
26. Cerebral Adrenoleukodystrophy Key Companies
27. Appendix
List of Top Selling Market Research Reports in 2023
https://www.delveinsight.com/report-store/alcoholic-hepatitis-market
https://www.delveinsight.com/report-store/b-cell-maturation-antigen-targeted-therapies-market
https://www.delveinsight.com/report-store/blastomycosis-market
https://www.delveinsight.com/report-store/chronic-pulmonary-infections-due-to-pseudomonas-aeruginosa-in-patients-with-cystic-fibrosis-market
https://www.delveinsight.com/report-store/c-x-c-chemokine-receptor-inhibitors-competitive-landscape-market-and-pipeline-analysis
https://www.delveinsight.com/report-store/digestive-system-fistula-market
https://www.delveinsight.com/report-store/fertility-monitoring-devices-fertility-testing-devices-market
https://www.delveinsight.com/report-store/hay-fever-conjunctivitis-market
https://www.delveinsight.com/report-store/kawasaki-disease-market
https://www.delveinsight.com/report-store/myopia-progression-market
https://www.delveinsight.com/report-store/pecoma-market
https://www.delveinsight.com/report-store/pediatric-growth-hormone-deficiency-market
https://www.delveinsight.com/report-store/percutaneous-arterial-closure-device-market
https://www.delveinsight.com/report-store/vital-sign-monitors-external-remote-patient-monitoring-devices-market
https://www.delveinsight.com/report-store/upper-tract-urothelial-cancer-market
https://www.delveinsight.com/report-store/varicose-vein-treatment-devices-market
https://www.delveinsight.com/report-store/uk-healthcare-outlook-report
https://www.delveinsight.com/report-store/supraventricular-tachycardia-market
https://www.delveinsight.com/report-store/stem-cell-market
https://www.delveinsight.com/report-store/soft-tissue-defect-market
https://www.delveinsight.com/report-store/small-lymphocytic-lymphoma-market
https://www.delveinsight.com/report-store/shigella-infections-market
https://www.delveinsight.com/report-store/ranibizumab-biosimilars-insight
https://www.delveinsight.com/report-store/radiation-retinopathy-market
https://www.delveinsight.com/report-store/primary-biliary-cholongitis-pbc-market
About Us
DelveInsight is a Business Consulting and Market research company, providing expert business solutions for the healthcare domain and offering quintessential advisory services in the areas of R&D, Strategy Formulation, Operations, Competitive Intelligence, Competitive Landscaping, and Mergers & Acquisitions.
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email: ybhardwaj@delveinsight.com
Phone: 9193216187
Address: 304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release A New Era in Cerebral Adrenoleukodystrophy Treatment: DelveInsight's Cerebral Adrenoleukodystrophy Clinical Trials Report Highlights a Pathway to Improved Therapies | Minoryx Therapeutics, POXEL SA, Orpheris Inc, Bluebird Bio Inc, Magenta Therapeutics Inc here
News-ID: 3142784 • Views: …
More Releases from DelveInsight Business Research
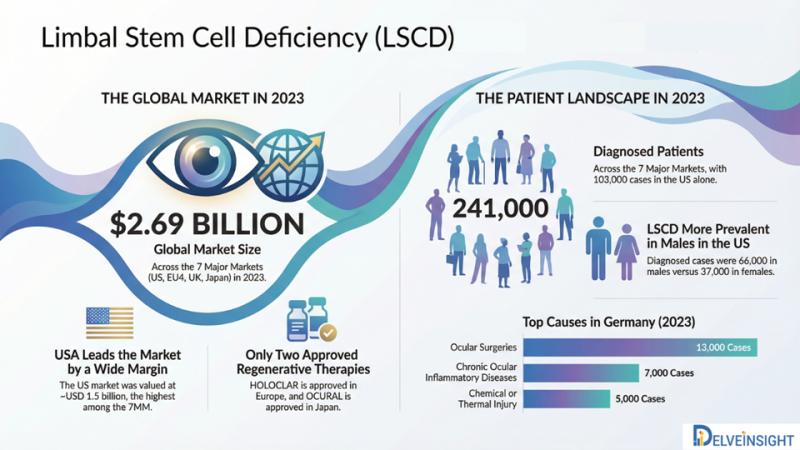
Limbal Stem Cell Deficiency Market to Surpass USD 2.6 Billion by 2034, Driven by …
In 2023, the Limbal Stem Cell Deficiency (LSCD) market was dominated by the United States, generating nearly USD 1.5 billion in revenue, while Spain represented the smallest market with approximately USD 127 million. This regional distribution is expected to remain consistent throughout the forecast timeline. The US accounted for nearly 103,000 diagnosed LSCD cases, whereas Japan recorded around 37,000 cases, with both countries projected to witness notable growth in patient…
SSc-ILD Market Set to Cross USD 750 Million by 2034, Driven by 10+ Emerging Ther …
The major players operating in the Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) market include Roche, Prometheus Biosciences, Inc., Merck, GlaxoSmithKline, Genentech, Inc., Acceleron, Boehringer Ingelheim, Actelion, Hôpital Claude-Huriez, Changchun GeneScience Pharmaceutical, among others.
DelveInsight's report titled "Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Forecast-2034" delivers a comprehensive analysis of SSc-ILD, covering historical data, projected epidemiology, and evolving market trends across the United States, EU4 (Germany, Spain, Italy, and France),…
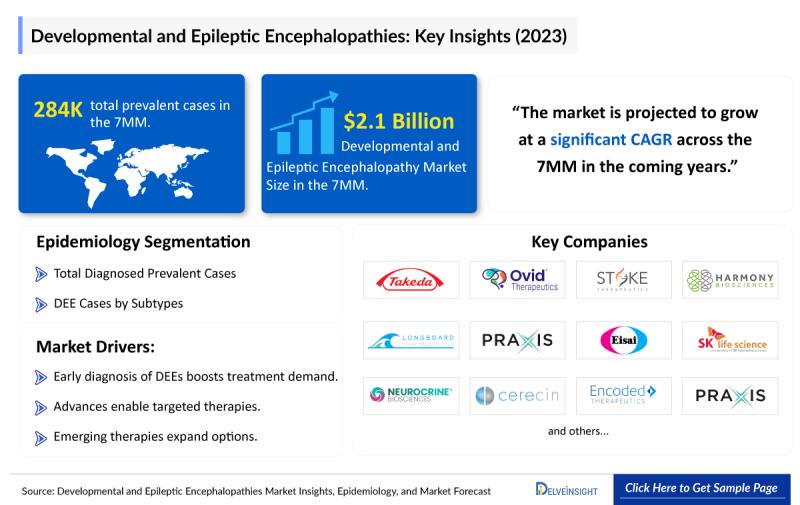
Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerat …
The Developmental and Epileptic Encephalopathy (DEE) treatment market across the seven major markets (7MM) was valued at nearly USD 2.1 billion in 2023 and is expected to register a healthy compound annual growth rate over the forecast period. The United States emerged as the largest contributor, capturing close to 80% of the overall market revenue.
The DEE treatment landscape is undergoing a significant transformation as the limitations of traditional antiepileptic drugs…
Polycythemia Vera Pipeline and Drug Development in 2025: 10+ Therapies and 8+ Co …
DelveInsight's "Polycythemia Vera Pipeline Insight 2025" report delivers an in-depth overview of the Polycythemia Vera pipeline landscape, covering more than 8 companies and 10+ pipeline candidates. The report analyzes both clinical-stage and preclinical assets, offering detailed drug profiles across various stages of development. It also evaluates Polycythemia Vera therapies based on product classification, development stage, route of administration, and molecular category, while additionally spotlighting inactive or discontinued pipeline assets.
Explore the…
More Releases for Cerebral
The Increasing Prevalence Of Cerebral Palsy : Core Growth Enabler in the Cerebra …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
What Will the Cerebral Palsy Treatment Industry Market Size Be by 2025?
In the past few years, the market size of cerebral palsy treatment has seen consistent growth. It is expected to increase from $3.51 billion in 2024 to $3.66 billion in 2025, demonstrating a compound annual growth rate…
Cerebral Palsy Market - Unleashing Potential: Breakthrough Therapies Shaping the …
Newark, New Castle, USA: The "Cerebral Palsy Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cerebral Palsy Market: https://www.growthplusreports.com/report/cerebral-palsy-market/7988
This latest report researches the industry structure, sales, revenue,…
Cerebral Palsy Market - Driving Breakthroughs in Cerebral Palsy Care: Redefining …
Newark, New Castle, USA - new report, titled Cerebral Palsy Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Cerebral Palsy market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Cerebral Palsy market. The report offers an overview of the market, which…
Cerebral Palsy Therapeutics Clinical Trials & Results
In cerebral palsy, the term cerebral refers to brain and palsy refers to the loss or impairment of motor function. Cerebral palsy is a group of neurological disorders that appear in infancy or early childhood, and permanently affect body movement and muscle coordination.
Download the sample report @ https://www.pharmaproff.com/request-sample/1093
It is caused by damage or abnormalities inside the developing brain that disrupt its ability to control movement and maintain posture and balance.…
Rising Prevalence of Cerebral Malaria and Increasing Novel Drug Treatment Drives …
Malaria is a mosquito-borne infectious disease affecting humans and animals caused by parasitic protozoan belonging to the plasmodium family. Malaria is typically transmitted from human to human through the bite of an infected Anopheles (female) mosquito. Symptoms of malaria include fever, headache, fatigue, muscle pain, diarrhea, anemia, and vomiting. Severe malaria can cause yellow skin, seizures, coma, or death. Cerebral malaria is a form of severe malaria. Cerebral malaria has…
Cerebral Palsy - Pipeline Review, H1 2017
ReportsWorldwide has announced the addition of a new report title Cerebral Palsy - Pipeline Review, H1 2017 to its growing collection of premium market research reports.
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Cerebral Palsy - Pipeline Review, H1 2017, provides an overview of the Cerebral Palsy (Central Nervous System) pipeline landscape.
Cerebral palsy is a group of disorders that can involve brain and nervous system functions, such as…
