Press release
100th EXCOR® Implantation in Spain
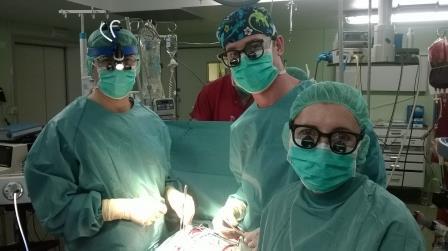
In March 2015 the clinic La Paz Madrid recorded a particular success: After ten years of EXCOR® therapy in Spain, the 100th implantation of the Berlin Heart was performed. La Paz is one of the leading Spanish hospitals for pediatric heart surgery. Almost simultaneously in another clinic in Madrid the 101st implantation took place and further implantations followed.The clinics in Spain benefit from an excellent service by Palex Medical SA as partner of Berlin Heart on the Iberian Peninsula. Every surgical procedure is supported by a team of clinical specialists on-site to make sure that all decisions meet the patient’s individual needs. The users are located in the seven most important cardio-surgical centers all over the country and thus offer general coverage.
The Spanish hospitals look back on a very positive EXCOR® experience, even in international comparison: 60 % of the patients could be successfully transplanted, 12 % are currently on EXCOR® support and hope for a donor heart. Due to the lack of organ donors, the current development in Spain, as in other countries, leads to more implantations of VADs. Together with Berlin Heart, Palex Medical SA is very proud to make an important contribution in saving lives and will further expand its ambitious service.
Berlin Heart GmbH is the only company worldwide that develops, produces, and distributes implantable and external ventricular assist devices for patients of every age and body size. The devices INCOR®, EXCOR® Adult and EXCOR® Pediatric can support failing hearts short-, mid- and long-term and therefore offer a life-saving therapy. Customers moreover benefit from clinical and technical support by Berlin Heart VAD specialists who are available 24/7. INCOR® and EXCOR® Adult are not FDA-approved, but widely used in Europe.
Berlin Heart GmbH
Katharina Schubert
Marketing & PR
Wiesenweg 10
12247 Berlin
schubert@berlinheart.de
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release 100th EXCOR® Implantation in Spain here
News-ID: 309978 • Views: …
More Releases from Berlin Heart

The 1,500th child supported by the Berlin Heart ventricular assist device has be …
The EXCOR® Pediatric ventricular assist device (VAD) has been implanted in more than 1,500 children worldwide to save their lives.
In June 2014 for the 1,500th time, the EXCOR® Pediatric device was implanted to save the life of a child: a 14-year-old boy from Hong Kong who was suffering from dilated cardiomyopathy since the age of four was supported with the life-saving device. Up to that point the teenager had…
More Releases for EXCOR®
1. Japanese EXCOR® Pediatric patient successfully transplanted
Berlin, 19.06.2013: The wait for a donor heart is over for the 1st Japanese patient treated with the EXCOR® Pediatric. On May 20th, 2013 the two-year-old girl was successfully transplanted at Morgan Stanley Childrens Hospital, New York Columbia Presbyterian Medical Center. According to the patient’s physicians, she tolerated the procedure well and is recovering as expected.
In August 2012, for the first time in Japan’s history, it was possible…
Longest support time with the EXCOR® Pediatric ventricular assist device
Six year old Lara from Erlangen is the toddler with the longest support time of a ventricular assist device (VAD) in the world.
The small patient was treated an entire 877 days with the VAD EXCOR® Pediatric in the University Hospital in Erlangen and, thanks to this therapy, could recover so well that she was now able to receive a new heart a few days ago.
In February 2010 Lara was in…
Major Milestone Achieved – EXCOR® Pediatric HDE Application Under Review
The Berlin Heart Group reported today that the results of the US IDE Trial for the Berlin Heart EXCOR® Pediatric Ventricular Assist Device have been submitted to the FDA in an HDE application. The FDA has informed the company that the application is officially under review and that a tentative panel date has been scheduled.
The study, which enrolled the first patient in November 2007, is the first prospective clinical…
Major Milestone Achieved – EXCOR® Pediatric HDE Application Under Review
Berlin Germany 12 April 2011: The Berlin Heart Group reported today that the results of the US IDE Trial for the Berlin Heart EXCOR® Pediatric Ventricular Assist Device have been submitted to the FDA in an HDE application. The FDA has informed the company that the application is officially under review and that a tentative panel date has been scheduled.
The study, which enrolled the first patient in November 2007,…
Successful transatlantic EXCOR® air ambulance transportations
Berlin, 30 March 2011. Four young children suffering from severe heart failure and consequently supported by the EXCOR® ventricular assist device, were safely and successfully transported by aircraft during the last months.
The so-called artificial heart was implanted in order to assist the patient’s heart function of pumping blood through the body. As a life-saving device, it bridges the time until a donor heart is found and transplantation becomes possible.…
Swedish clinics achieve excellent outcome with EXCOR® assist device program
It is only three years ago, when the Sahlgrenska University Hospital and the Queen Silvia Children Hospital in Sweden started to implant the EXCOR® ventricular assist device (VAD) to patients suffering from severe heart failure. In the event of the own heart no longer being able to provide the vital pump function of pumping blood through the body, the device offers a life-saving therapy for patients of every age and…