Press release
Saliva test instead of blood test
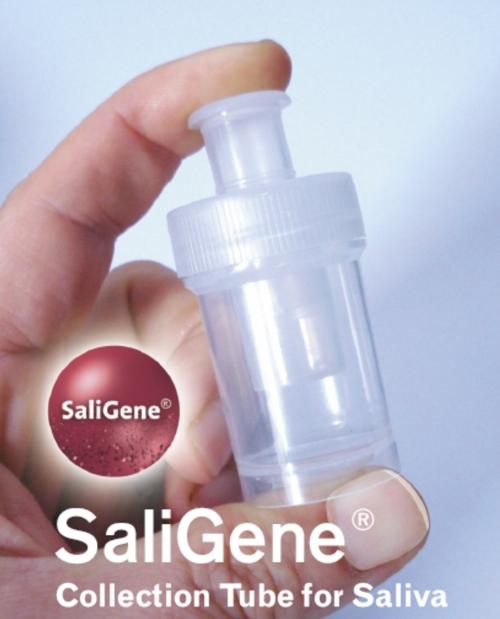
New product – SaliGene® Collection TubeThe SaliGene® Collection Tube is the easiest way to collect and preserve large amounts of DNA from saliva. It is easy-to-use and completely non-invasive, making it ideal for reliable self-administered collection. As part of the PSP® Spin Saliva DNA Kit SaliGene® features an all-in-one system for the collection, preservation, transportation and purification of DNA from saliva. Samples do not require special handling or storage because the DNA is stable for weeks at room temperature in the special DNA stabilizer solution. DNA from SaliGene® performs the same as DNA from blood, but there is no painful blood withdrawl. Therefore this system is highly suitable for children and needle-phobic people. Furthermore the risk of infection with blood-bourne viruses like HIV and Hepatitis is eliminated. The isolated DNA is fully functional for demanding downstream applications and can be processed using high-throughput purification robots. SaliGene® is a perfect tool for genotyping (forensic analysis, paternity testing), pharmacogenetics and molecular diagnostics (e.g. mutation screening) .
Invitek Gesellschaft für Biotechnik & Biodesign mbH
Robert-Rössle-Str. 10
D - 13125 Berlin
Germany
Sonja Farhangi
phone: +49 30 9489 3796
fax: +49 30 9489 3795
marketing@invitek.de
www.invitek.de
INVITEK BIOTECHNOLOGY & BIODESIGN LTD. is a worldwide acting provider of innovative, high-quality solutions and technical support to the life sciences and diagnostics industry. Since 1992 the company is internationally acclaimed for its outstanding and high performance technology platform and offers a broad spectrum of superior products for nucleic acid purification, molecular diagnostics, drug discovery and biomedical research. Within the modular product concept “I-tek solution”, Invitek combines its technological developments in complete solutions for molecular diagnostics. An example is the successful introduction of an early warning system for colon carcinoma. Invitek has been certified under EN ISO 9001: 2000 and EN ISO 13485 : 2003 and produces under EC Directive 98/79/EEC (IVD-MD).
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Saliva test instead of blood test here
News-ID: 30472 • Views: …
More Releases from Invitek Biotechnology & Biodesign Ltd.

Invitek concludes strategic alliance with Chinese partners
In November 2007 a master agreement between Invitek Biotechnology & Biodesign Ltd., Allrun Nano Science & Technology Co., Ltd. and Research Institute of Micro/Nano Science & Technology in Shanghai Jiao Tong University was signed at the 3rd International Nanotechnology Cooperation Symposium in Shanghai, China. Within this agreement the stragetic cooperation for development of new and innovative separation procedures of biomolecules (nucleic acids, proteins) and cells as well as of novel…

New dimension in automation of nucleic acid purification and molecular diagnosti …
Invitek Biotechnolgy & Biodesign Ltd. announces the introduction of a new generation of superparamagnetic nanoparticles for nucleic acid isolation (InviMag® nano). In cooperation with the Chinese company Allrun Nano Science & Technology CO., Ltd. Invitek will adapt and extend its product portfolio for manual and fully automated magnetic extraction of nucleic acids during autumn 2007. The high-performance paramagnetic nanobeads with a controlled surface featuring various functionalities are applicable for efficient…
Personalised medicine: milestone in the therapy control of humanised antibodies
CENiMED GmbH, BioTeZ Berlin-Buch GmbH and Invitek Gesellschaft für Biotechnik & Biodesign mbH (company for biotechnology and bio-design) announce their cooperation in the development, production and marketing of Recovery-ELISAs for the therapy control of a new group of therapeutics, the so called humanised antibodies.
Humanised antibodies offer unforeseen potentialities in cancer therapy, e. g. for the treatment of mamma carcinoma. Previous therapy control methods, however, could not avoid overdoses which…
More Releases for SaliGene®
Punch!® Software Releases Punch!CAD™ SharkCAD® & ViaCAD® 15
NOVATO, Calif., December 10, 2024 - Punch!® Software, a leading software developer of Home Design and CAD titles for over 25 years, today announced the release of new Windows and Mac versions of the SharkCAD® 15 and ViaCAD® 15 family of products.
This latest release encompasses a range of variants for the Windows and Macintosh platform: SharkCAD® Pro, SharkCAD®, ViaCAD® Pro, ViaCAD® 2D/3D, and ViaCAD® 2D.
Punch!CAD™ products continue to provide innovative…
Cherrywork® Accounts Payable Automation by Incture® Now Available on SAP® Sto …
Incture® today announced the availability of Cherrywork® Accounts Payable Automation for purchase on SAP® Store, the online marketplace for SAP and partner offerings. Cherrywork® Accounts Payable Automation is built on SAP Business Technology Platform (SAP BTP) to streamline end-to-end accounts payable operations, reduce accounting costs, and help increase cash flow for customers.
"Cherrywork Accounts Payable Automation is the hyperautomation solution to step up the source to pay process for our customers.…
Ultimate Chemicals® Featured in Manufacturing Marvels® on The Fox Business Net …
MOORE, OK., Oct. 8, 2021/ -- Ultimate Chemicals® Inc., an integrated manufacturer and supplier of chemicals and related services, is pleased to announce it will be showcased in a broadcast of Manufacturing Marvels® scheduled to air on October 14, 2021 at approximately 9:30-9:44 p.m. EST on The Fox Business Network® (FBN).
Ultimate Chemicals has created their products to help restore surfaces to their optimum surface capability. Non-destructive to the surfaces to…
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA and Intelligent Enterprise to Manufacturing, Consumer Products, Higher education, and research, Industrial machinery and components, Life sciences and Automotive Industries
Chicago, IL, June 10, 2020 – Sunera Technologies, Inc announced that it had joined the SAP® PartnerEdge® program as a Re-sell & Services partner, through which it will resell SAP solutions to midsize organizations in North America. Through this engagement, SuneraTech…
GRAMMY® Performers Receiving AnthroSpa Logic® Announced
CHICAGO, IL- February, 2011- The Performers at the 53rd Annual GRAMMY® Awards, who will receive AnthroSpa Logic®, a luxury, 100% natural spa skin care and body care line inspired by the science of anthropology have been announced. The unique skin care line combines beauty secrets from around world and will be included in the in the Official VIP “Gift Bag” for the event, which takes place on Feb. 13,…
American Megatrends Announces AMIBIOS®8 & Aptio®4.x Support for the Intel® Xe …
ATLANTA, GEORGIA - American Megatrends (AMI) is pleased to announce that its AMIBIOS®8 & Aptio®4.x products feature support for the new Intel® Xeon® 5500 Platform.
The Intel® Xeon® 5500 platform combines the Intel® Xeon® Processor 5500 Series with the Intel® 5520 Chipset for embedded, storage, security and communications infrastructure applications in a wide range of form factors. There are four different processor options for this platform with seven year lifecycle support…