Press release
Global GMP Storage Market: Trends, Drivers, and Key Players in Pharmaceutical Logistics and Supply Chain Management
The GMP storage market refers to the global industry involved in providing storage solutions that comply with Good Manufacturing Practices (GMP) regulations. GMP regulations are guidelines that ensure pharmaceuticals, biopharmaceuticals, medical devices, and other healthcare products are consistently produced and controlled according to quality standards.Key players in the GMP storage market include major logistics and transportation companies such as DHL, UPS, and FedEx, as well as specialized healthcare logistics providers such as Marken, World Courier, and QuickSTAT. The market is highly competitive, with companies competing on factors such as facility design and capabilities, technology and automation, and compliance with regulatory requirements.
Click Here to Get a Sample Copy of this Report@:
https://www.persistencemarketresearch.com/samples/33113
The GMP storage market is driven by several factors, including the increasing demand for pharmaceutical and biopharmaceutical products, the rising need for specialized storage solutions that comply with regulatory requirements, and the growing trend towards outsourcing of pharmaceutical logistics and supply chain management.
GMP storage facilities are critical components of the pharmaceutical and biopharmaceutical supply chain. These facilities provide secure, temperature-controlled storage for materials and products that require specific environmental conditions, such as refrigeration, freezing, or humidity control. They are designed to minimize the risk of contamination, cross-contamination, and other quality-related issues that could compromise product efficacy and patient safety.
You Can Customize this Report as per Your Requirement Click Here@:
https://www.persistencemarketresearch.com/request-customization/33113
The GMP storage market is expected to continue growing in the coming years, driven by factors such as the increasing demand for specialized storage solutions that comply with regulatory requirements, the growing trend towards outsourcing of pharmaceutical logistics and supply chain management, and the increasing adoption of advanced technologies and automation in storage and logistics operations. However, the market may also face challenges such as regulatory pressures, quality-related issues, and pricing pressures from competitors.
What GMP Storage Product Kind Produces the Most Income?
"High Demand Across Regions for Biologics Research & Production"
In 2021, the GMP storage goods market represented a share of about 83.1% of the overall GMP storage market.
The use of biomedical refrigerators and freezers is increasing, which is accelerating market growth. These factors include the expansion of the biotechnology and life science industries in developed regions (North America and Europe) and the rising demand for the development and manufacture of biological products that need to be refrigerated or frozen.
Here are Some Related Research Reports
CAR T-Cell Therapy Market
https://www.persistencemarketresearch.com/market-research/car-t-cell-therapy-market.asp
Franz Cell and Vapometer Market
https://www.persistencemarketresearch.com/market-research/franz-cell-and-vapometer-market.asp
Synthetic Biology Market
https://www.persistencemarketresearch.com/market-research/synthetic-biology-market.asp
Contact
Rajendra Singh
Persistence Market Research
U.S. Sales Office: 305 Broadway, 7th Floor New York City,
NY 10007 +1-646-568-7751 United States
USA - Canada Toll-Free: 800-961-0353
About Persistence Market Research:
Persistence Market Research is always way ahead of its time. In other words, it tables market solutions by stepping into the companies'/clients' shoes much before they themselves have a sneak pick into the market. The pro-active approach followed by experts at Persistence Market Research helps companies/clients lay their hands on techno-commercial insights beforehand, so that the subsequent course of action could be simplified on their part.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global GMP Storage Market: Trends, Drivers, and Key Players in Pharmaceutical Logistics and Supply Chain Management here
News-ID: 2947497 • Views: …
More Releases from Persistence Market Research
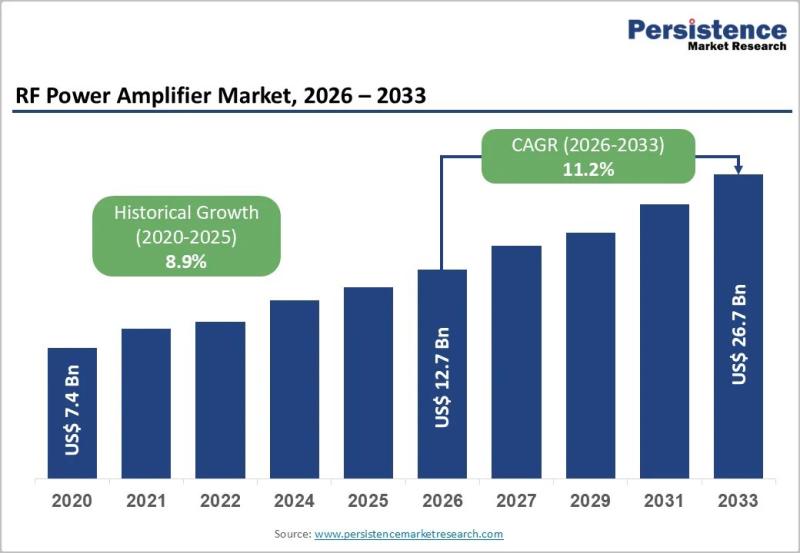
RF Power Amplifier Market Accelerates on 5G, GaN, and Defense Modernisation
RF Power Amplifier Market Overview and Growth Outlook
The global RF Power Amplifier Market is poised for sustained double-digit growth, projected to rise from US$ 12.7 billion in 2026 to US$ 26.7 billion by 2033, reflecting a strong CAGR of 11.2% during the forecast period. This expansion is underpinned by structural demand across telecommunications infrastructure, aerospace and defense modernization, and next-generation satellite communication (SATCOM) systems. As mobile data traffic surges and…
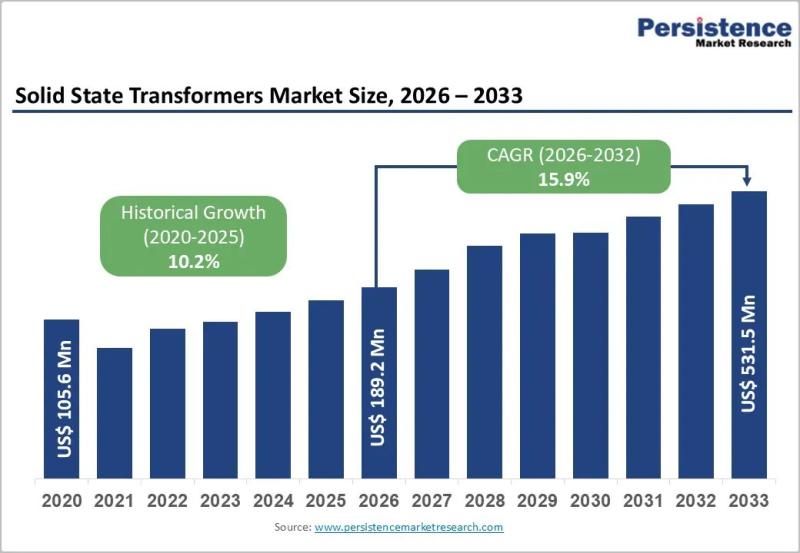
Smart Grid Expansion Driving Solid State Transformers Demand
Solid State Transformers Market Overview and Growth Outlook
The global Solid State Transformers Market is entering a high-growth phase, driven by rapid grid modernization and the accelerating shift toward renewable energy. Valued at US$ 189.2 million in 2026, the market is projected to reach US$ 531.5 million by 2033, expanding at a robust CAGR of 15.9% during the forecast period. The rising need for intelligent power distribution, bidirectional energy flow, and…
Enterprise Password Management Market to Reach US$ 9.4 Billion by 2033 as Zero-T …
The Enterprise Password Management Market is expanding rapidly as organizations confront escalating cyber threats, data breach risks, and complex regulatory mandates. Valued at US$ 3.2 billion in 2026, the market is projected to reach US$ 9.4 billion by 2033, registering a strong CAGR of 16.8% between 2026 and 2033. This accelerated growth reflects rising investment in identity security infrastructure as enterprises adopt zero-trust frameworks and strengthen credential governance.
Historically, the market…
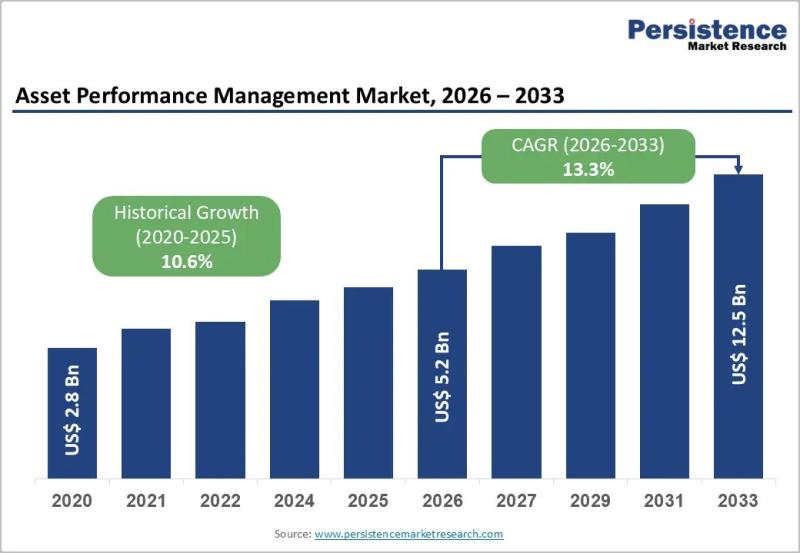
Asset Performance Management Market to Reach US$ 12.5 Billion by 2033 as AI-Driv …
Overview of the Asset Performance Management Market
The Asset Performance Management (APM) Market is witnessing strong acceleration as industries prioritize predictive maintenance, reliability engineering, and lifecycle optimization to reduce operational risks. Valued at US$ 5.2 billion in 2026, the global market is projected to reach US$ 12.5 billion by 2033, expanding at a CAGR of 13.3% between 2026 and 2033, compared to a historical CAGR of 10.6% from 2020 to 2026.…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
