Press release
Increasing Investments On Bio-Tech And Pharma Research And Development (R&D) Activities, Lucrative Opportunity Of The Inspection Machines Market - FMI
The global inspection machines market is expected to bolster over the projection with a robust CAGR of 5.1% over the forecast period from 2022 to 2032. The global market is anticipated to be appraised at US$ 1,053.5 Mn by 2032, up from US$ 643.1 Mn in 2022. It has been observed that a surging number of inspection checkpoints are present throughout the production line. This is likely to drive the sales of inspection machines. Although, integrating inspection equipment into the manufacturing and packaging line is difficult because of the complexity of machines and the heightened end-use environment. Conversely, increasing investments in biotech and pharma research and development (R&D) activities are anticipated to generate remunerative opportunities for the market.Several research studies, along with various product development and launches are anticipated to surge the growth of the inspection machines market trends. A new VT-X750-V3 system, which is considered to be the fastest CT-type X-ray inspection device, was released by OMRON Corporation in 2021. Other market players launched X-ray technologies OEM product range for industrial and pre-clinical X-ray imaging systems.
Over the recent years, a new concept of the non-destructive testing device with the help of a novel carbon nanotube (CNT) based miniature X-ray tube has been introduced recently. Such advancement is estimated to increase the adoption of inspection machines since they can be used for small-scale internal inspection of objects.
Request a Report Sample @ https://www.futuremarketinsights.com/reports/sample/rep-gb-2132
Key Takeaways
- Product recalls have been surging due to unearthed issues including such cases when it is defective or at risk of health or both. Furthermore, rising regulatory compliance with good manufacturing practices (GMP) is contributing to the presence of major governmental and non-governmental authorities that inspect the final products deployed in the market by manufacturers. These factors are expected to propel the demand for inspection machines.
- Within the pharmaceutical sector, several regulatory agencies such as the Food and Drug Administration (FDA), European Medicines Agency, and the Pharmaceuticals and Medical Devices Agency carefully monitor the compliance of manufacturers with Current Good Manufacturing Practice (CGMP) regulations to ensure the quality of drugs and medical devices. These regulations are important as they ensure product safety, and the claims of ingredients provided are verifiable.
- The prominence of CMOs and CROs has surged in manufacturing and packaging due to the possibility of reducing timeframes with simultaneously offering external validation and expertise. Since contract manufacturers have specialized teams that can efficiently grasp client quality standards are now known to be a valuable source for OEMs. These aforementioned factors are likely to accelerate the growth of the inspection machines market share.
- Developing markets have witnessed a surge within the unorganized healthcare systems. Due to this, the lack of adequate infrastructure and poverty limit the expansion of the industry. Additionally, there has been an increase in the demand for refurbished machines, although these machines are expensive. These factors are expected to hinder the global market growth.
Competitive Landscape
The inspection machines market share is predicted to increase as manufacturers are searching to expand their production and presence all over the market through several strategic tactics such as various collaborations and product launches. Furthermore, the industry is becoming highly competitive amongst market players in terms of product variation and pricing.
Browse Full Reports@ https://www.futuremarketinsights.com/reports/inspection-machines-market
More Insights into the Inspection Machines Market
North America is expected to witness lucrative growth opportunities over the projection period by accounting for a total revenue of around 23.5%. This has been attributed to several types of research that have been conducted over the years based on the importance of the inspection of devices during manufacturing.
Sound manufacturing practices must be inherently built right into the manufacturing process since they are unable to get tested on individual batches of products. Therefore, the inspection machines market growth is anticipated to propel attributing to such research to emphasize the importance of GMP.
Prominent market players are focusing on introducing technologically advanced gadgets with systems such as the In-Sight 3D-L4000 embedded vision system. It allows engineers to be quicker and more accurate and cost-effectively solve a range of inspections on automated production lines through the implementation of 3D laser displacement technology.
Owing to rising technological advancements, recently, a new concept for a non-destruction device has emerged which is based on an X-ray tube. With the help of carbon nanotube-based miniature x-ray tubes, such devices are gaining traction in the global inspection machines market. These technological advancements are expected to bolster market growth over the projection period.
Contact Us:
Future Market Insights, Inc
Unit No: 1602-006
Jumeirah Bay 2
Plot No: JLT-PH2-X2A
Jumeirah Lakes Towers
Dubai
United Arab Emirates
For Sales Enquiries: sales@futuremarketinsights.com
Browse All Reports: https://www.futuremarketinsights.com/reports
About Future Market Insights (FMI)
Future Market Insights (ESOMAR certified market research organization and a member of the Greater New York Chamber of Commerce) provides in-depth insights into governing factors elevating the demand in the market. It discloses opportunities that will favour the market growth in various segments based on Source, Application, Sales Channel and End-Use over the next 10 years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Increasing Investments On Bio-Tech And Pharma Research And Development (R&D) Activities, Lucrative Opportunity Of The Inspection Machines Market - FMI here
News-ID: 2859390 • Views: …
More Releases from Future Market Insights
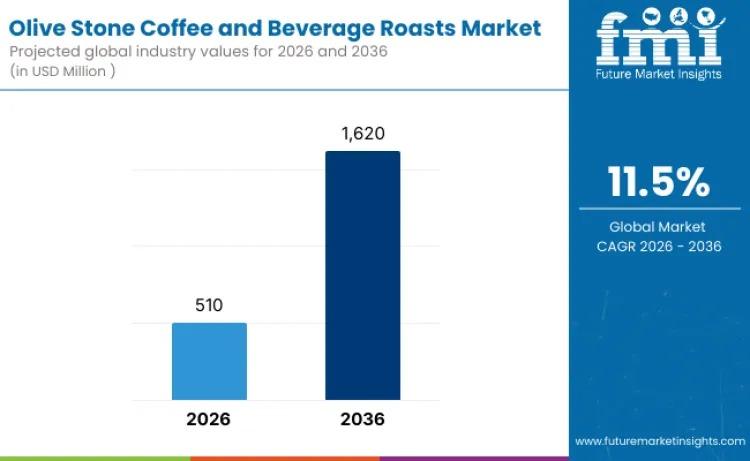
Global Olive Stone Coffee and Beverage Roasts Market to Reach USD 1,620 Million …
The global olive stone coffee and beverage roasts market is entering a high-growth decade, fueled by sustainability innovation and evolving specialty coffee culture. Valued at USD 510 million in 2026, the market is projected to reach USD 1,620 million by 2036, expanding at a compelling CAGR of 11.5%.
As consumers increasingly seek beverages that combine sustainability, functionality, and distinctive taste, olive stone-based roasting solutions are transitioning from niche experimentation to structured…
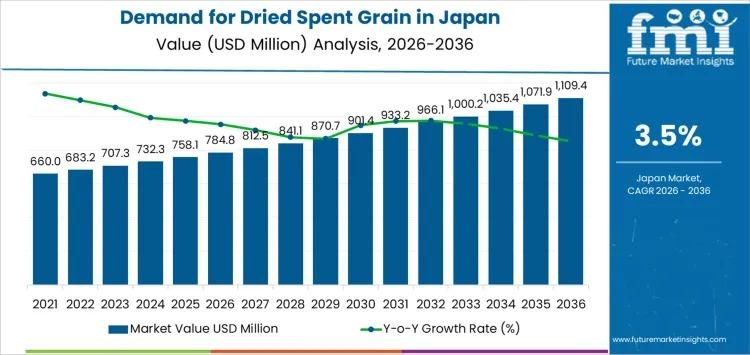
Japan Dried Spent Grain Market to Surpass USD 1.1 Billion by 2036 as Feed Optimi …
Japan's dried spent grain market is entering a decade of steady, value-driven expansion, supported by structured feed demand, brewery byproduct utilization, and rising integration of fiber-rich ingredients into food manufacturing. Industry estimates place the market at USD 784.8 million in 2026, with projections indicating growth to USD 1,109.4 million by 2036, reflecting a CAGR of 3.5%.
Between 2020 and 2026, demand increased from USD 637.5 million to USD 784.8 million, shaped…
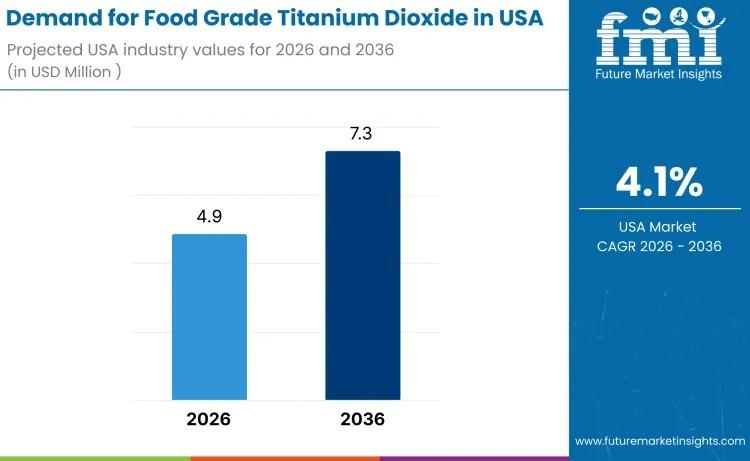
USA Food Grade Titanium Dioxide Market to Reach USD 7.3 Million by 2036 Amid Ste …
The demand for food grade titanium dioxide in the USA is valued at USD 4.9 million in 2026 and is projected to reach USD 7.3 million by 2036, expanding at a CAGR of 4.1%. Growth remains moderate yet stable, supported by continued use of titanium dioxide as a whitening and opacifying agent across confectionery coatings, bakery decorations, sauces, dairy analogues, and processed food matrices.
Despite heightened regulatory scrutiny and evolving clean-label…
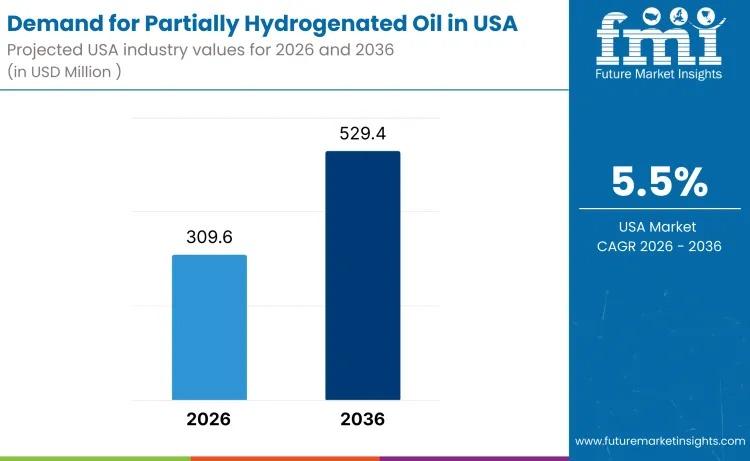
USA Partially Hydrogenated Oil Market to Reach USD 529.4 Million by 2036 Amid Me …
The demand for partially hydrogenated oil in the USA is projected to rise from USD 309.6 million in 2026 to USD 529.4 million by 2036, expanding at a steady CAGR of 5.5%. While edible applications remain tightly regulated, demand persists across specialty industrial and permitted food-related segments where oxidative stability, viscosity control, and texture performance remain critical.
Despite regulatory constraints on trans fats in conventional food manufacturing, PHOs continue to serve…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
