Press release
Competitive Insurance Pricing to be the focal point of the Infectious Disease In-Vitro Diagnostics Market at a CAGR of 4.4%
The global Infectious Disease In-Vitro Diagnostics Market was valued at around US$ 58.7 Bn in 2021. With a projected CAGR of 4.4% for the next ten years, the market is likely to reach a valuation of nearly US$ 93.9 Bn by the end of 2032. A window of opportunity has emerged for the early identification and control of infectious diseases such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), Ebola, chikungunya, avian flu, swine flu, and Zika due to their epidemiological burden. This has significant potential to boost Infectious Disease in In-Vitro Diagnostics.Sales Analysis of Infectious Disease In-Vitro Diagnostics Market from 2017 to 2021 Vs Market Outlook for 2022 to 2032
The global market for the Infectious Disease In-Vitro Diagnostics Market expanded at a CAGR of 4.4% over the last four years (2017-2021). The rising burden of infectious diseases across the globe has increased the demand for infectious disease diagnostics products. Around 70% of medical decisions are based on information contained in electronic medical records (IVDs), which is why they are an essential component of patient care. The majority of the IVD market is concentrated in developed countries, but the United States, Europe, and Japan account for over 80% of all global sales.
Get Sample Copy of Report @ https://www.persistencemarketresearch.com/samples/33205
The United States will continue to be the largest shareholder of the Infectious Disease In-Vitro Diagnostics Market throughout the analysis period accounting for over US$ 9.4 Bn absolute dollar opportunity in the coming 10-year period.
How are key drivers going to affect the Infectious Disease In-Vitro Diagnostics Market?
Around 10% of the world's GDP is constituted of global healthcare spending, which has been steadily rising in recent years as a result of the population's rising healthcare needs. Over 60% of clinical decisions are influenced by In-Vitro Diagnostics, which is important to the healthcare sector. However, it barely represents 2% of all healthcare costs. This suggests that there is a huge opportunity for in-vitro diagnostics that are affordable. This has been one of the key market drivers.
Government support is a key driver of growth for the In-Vitro Diagnostics market in the near future because increased funding from the government enables research institutions to create quick analysis systems that are helpful for diagnosing a variety of diseases using various samples. For instance, the European Union's "recovery plan" will invest over US$ 250 Bn by the end of 2026.
In-Vitro Diagnostics technologies were only applied in clinical labs in the past. The majority of clinical tests in the fields of chemistry, immunochemistry, and hematology are still carried out utilizing high throughput equipment with intricate automation. Where the patients are located, point of care (POC) testing is implemented and it is expanding quickly. The big manufacturing firms are concentrating on releasing quick, portable, easy-to-use tools that are affordable and suitable for usage outside of laboratories. The delocalization of the diagnostics offers, including auto-diagnostics and home diagnostics, is anticipated to be improved by this need.
Download TOC, list of figures & tables @ https://www.persistencemarketresearch.com/market-research/infectious-disease-in-vitro-diagnostics-market/toc
Competitive Landscape
Market participants are tying with sister companies to combine new innovative products. The key companies operating in the Infectious Disease In-Vitro Diagnostics Market include Abbott Laboratories, Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd., bioMérieux SA, Siemens Healthineers AG, Danaher Corporation, Becton Dickinson and Company, PerkinElmer, Inc., Hologic, Inc., QIAGEN N.V., Grifols S.A., DiaSorin S.p.A, Bio-Rad Laboratories, Inc., Sysmex Corporation, Ortho Clinical Diagnostics Holdings plc, Quidel Corporation, Meridian Bioscience, Genetic Signatures Ltd., OraSure Technologies, Trinity Biotech Plc., Chembio Diagnostic Systems, Seegene, Inc., Co-Diagnostics, ELITechGroup, Epitope Diagnostics, Trivitron Healthcare, Meril Life Sciences Pvt. Ltd., InBios International, Vela Diagnostics, and Uniogen Oy.
Some of the recent developments by key providers of the Infectious Disease In-Vitro Diagnostics Market are as follows:
In February 2019, Abbott was awarded CE Mark for DETERMINE HBSAG 2 test for the detection and diagnosis of Hepatitis B surface antigen.
In March 2021, a merger agreement between GenMark diagnostics Inc. and F. Hoffmann-La Roche Ltd. With just one patient sample, Roche will now have larger access to GenMark's cutting-edge technology for testing for a variety of infections.
In March 2021, in nations that recognize the CE mark, F. Hoffmann-La Roche Ltd. launched the Cobas pure integrated solution. Three technologies are combined on a single platform in this new compact analyzer, which makes operations in small to medium-sized labs easier.
Buy Full Report Now and Get Up to 20% Discount @ https://www.persistencemarketresearch.com/checkout/33205
Contact us:
Persistence Market Research
Address - 305 Broadway, 7th Floor, New York City,
NY 10007 United States
U.S. Ph. - +1-646-568-7751
USA-Canada Toll-free - +1 800-961-0353
Sales - sales@persistencemarketresearch.com
About us:
Persistence Market Research is here to provide companies a one-stop solution with regards to bettering customer experience. It does engage in gathering appropriate feedback after getting through personalized customer interactions for adding value to customers' experience by acting as the "missing" link between "customer relationships" and "business outcomes'. The best possible returns are assured therein.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Competitive Insurance Pricing to be the focal point of the Infectious Disease In-Vitro Diagnostics Market at a CAGR of 4.4% here
News-ID: 2841407 • Views: …
More Releases from Persistence Market Research

Event Tourism Market Set for Exponential Growth through 2032 - PMR Research
The global Event Tourism Market is poised for remarkable expansion, driven by sustained demand for live experiences, increased business travel, hybrid event adoption, and a rebound in international tourism. According to industry projections, the market is expected to grow from an estimated US$1,538.3 billion in 2025 to US$2,631.5 billion by 2032, registering a CAGR of 7.3% over the forecast period.
This robust growth underscores the evolution of event tourism into one…

Residential Lighting Fixtures Market Set for Robust Growth Through 2032
The global residential lighting fixtures market is poised for strong expansion over the coming decade, underpinned by shifting consumer preferences toward energy efficient, smart, and design centric lighting solutions. According to a comprehensive market analysis by Persistence Market Research, the market was valued at US$ 20.4 billion in 2025 and is projected to reach US$ 32.2 billion by 2032, growing at a CAGR of 5.9 % from 2025 to 2032.
➤…

Active Modified Atmospheric Packaging Market to Reach US$ 37.9 Billion by 2033 - …
The active modified atmospheric packaging market is gaining strong traction as food manufacturers retailers and logistics providers focus on extending product shelf life while maintaining freshness quality and safety. Active modified atmospheric packaging integrates gas flushing scavengers and moisture regulators to actively control the internal atmosphere of a package thereby slowing microbial growth oxidation and spoilage. Unlike passive packaging this advanced technology continuously interacts with the packaged product creating an…
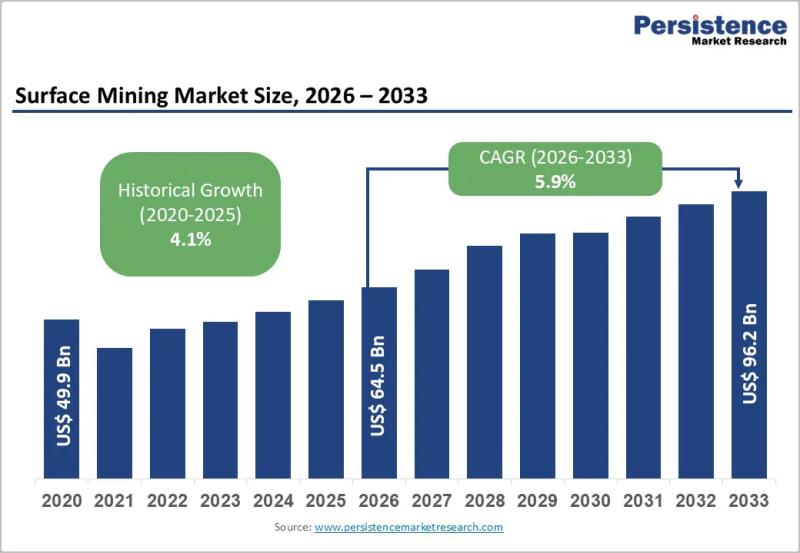
Surface Mining Market Size to Reach US$ 96.2 Billion by 2033 - Persistence Marke …
The surface mining market plays a critical role in the global extraction industry, enabling efficient recovery of minerals, metals, and fossil fuels located near the earth's surface. Surface mining techniques such as open pit mining, strip mining, mountaintop removal, and quarrying are widely used for coal, iron ore, copper, bauxite, and other industrial minerals. Compared to underground mining, surface mining offers higher production rates, lower operational risks, and improved cost…
More Releases for Diagnostic
Rubella Diagnostic Testing Market Report 2024 - Rubella Diagnostic Testing Marke …
"The Business Research Company recently released a comprehensive report on the Global Rubella Diagnostic Testing Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive…
Diagnostic Electrocardiograph Market - Elevating Cardiac Care: Diagnostic Electr …
Newark, New Castle, USA: The "Diagnostic Electrocardiograph Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Diagnostic Electrocardiograph Market: https://www.growthplusreports.com/report/diagnostic-electrocardiograph-market/7804
This latest report researches the industry structure, sales, revenue,…
Diagnostic Imaging Market - Empowering Precision Medicine: Harnessing the Potent …
Newark, New Castle, USA: The "Diagnostic Imaging Market" provides a value chain analysis of revenue for the anticipated period from 2021 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Diagnostic Imaging Market: https://www.growthplusreports.com/report/diagnostic-imaging-market/7688
This latest report researches the industry structure, sales, revenue,…
Rubella Diagnostic Testing Market - Advancing Rubella Control: Innovating Diagno …
Newark, New Castle, USA - new report, titled Rubella Diagnostic Testing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Rubella Diagnostic Testing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Rubella Diagnostic Testing market. The report offers an overview of…
Tenet Diagnostic: Reshaping the Diagnostic Industry in India
Tenet Diagnostic was incorporated in 2018 with an aim to establish high-quality diagnostics laboratories across India. The company caters to both B2B and B2C. They have seven different verticals namely: End Customer (B2C), Lab to Lab Test Outsourcing, Corporates and Institutions, large management (Hospitals, Medical Colleges, and other healthcare institutions), Clinical Trials, Home Sample Collection, and Government PPP Projects.
Tenet Diagnostics got its NABL certification in the fastest time and is…
Diagnostic Imaging Services Market 2019 Analyzed by Top Key Players PH3 Healthca …
Big Market Research has added a report, titled, "Diagnostic Imaging Services" The report not only provides a comprehensive analysis of market overview and dynamics for the historical period, 2019-2026, but also offers global and regional forecasts on market value, volume production, and consumption during the future period, 2019-2026. The report also analyzes the key market players, especially the distributors, along with the industrial chain structure. The evolution of market trends…