Press release
Medical Devices Regulatory Affairs Market Update- See How Industry Players are Preparing Against U.S. Breakdown Depression
Allied Market Research added new research on Global Medical Devices Regulatory Affairs Market- Global Opportunity Analysis and Industry Forecast, 2022-2030. The Medical Devices Regulatory Affairs market explores comprehensive study on various segments like size, share, development, innovation, sales and overall growth of major players. The research is based on primary and secondary data sources and it consists both qualitative and quantitative detailing. Some of the key players involved in the study are Accell Clinical Research, LLC., GenPact Ltd., Criterium, Inc., PRA Health Sciences, Promedica International, WuXi AppTec Inc., Medpace, Charles River Laboratories International, Inc., Icon plc, Parexel International Corporation, Freyr.Get Free Sample PDF of Medical Devices Regulatory Affairs Market Report>>> https://www.alliedmarketresearch.com/request-toc-and-sample/13738
Which market perspectives are enlightened in the Medical Devices Regulatory Affairs market report?
Executive Summary: It covers a summary of the most vital studies, the Worldwide Medical Devices Regulatory Affairs market increasing rate, modest circumstances, market trends, drivers and problems as well as macroscopic pointers.
Study Analysis: This covers major players, vital market segments, the scope of the products offered in the Medical Devices Regulatory Affairs market, the years measured and the study points.
Competitive Analysis: In this segment each player is screened based on a products, services, value, SWOT analysis, growth and other significant features.
Geographic Analysis: This Medical Devices Regulatory Affairs market report analyses data on the basis of production, sales, imports & exports, and key players in all regional markets.
"Medical Devices Regulatory Affairs most commonly refers to a biological technique that involves the use of light to control neurons that have been genetically modified to express light-sensitive ion channels. As such, Medical Devices Regulatory Affairs is a neuromodulator method that uses a combination of techniques from optics and genetics to control the activities of individual neurons in living tissue even within freely-moving animals. In some usages, Medical Devices Regulatory Affairs also refers to optical monitoring of neuronal activity and control of biochemical pathways in non-neuronal cells, although these research activities preceded the use of light-sensitive ion channels in neurons."
Medical Devices Regulatory Affairs Market Segments and Sub-segments::
Major Key Players: Accell Clinical Research, LLC., GenPact Ltd., Criterium, Inc., PRA Health Sciences, Promedica International, WuXi AppTec Inc., Medpace, Charles River Laboratories International, Inc., Icon plc, Parexel International Corporation, Freyr
Medical Devices Regulatory Affairs Market Segmentation by Service Provider: In-House, Outsourcing
Medical Devices Regulatory Affairs Market Segmentation by Service Type: Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Application, and Others
Medical Devices Regulatory Affairs Market Segmentation by Indication: Oncology, Neurology, Cardiology, Immunology, and Others
Medical Devices Regulatory Affairs Market Segmentation by Product Stage: Preclinical Trials, Clinical Trials, and Post Market Trials
Ask more about Medical Devices Regulatory Affairs Market Report>>> https://www.alliedmarketresearch.com/purchase-enquiry/13738
Interpretative Tools Used in Market Analysis: The methodical tools including SWOT analysis, Porter's five forces analysis, and investment return examination were used while breaking down the development of the key players performing in the market.
Growth Indicators in the Market: This section of the report covers the indicators that contains mergers & acquisitions, R&D, new product development, joint ventures, and associations of leading participants working in the market.
Key Questions Answered:
Who are the leading players involved in Medical Devices Regulatory Affairs Market?
Which are the major regions covered in Medical Devices Regulatory Affairs Market report?
Which is the leading revenue-generating region in Medical Devices Regulatory Affairs Market?
Which is the most influencing segment growing in the Medical Devices Regulatory Affairs market report?
What are the key trends in the Medical Devices Regulatory Affairs market report?
What is the total market value of Medical Devices Regulatory Affairs market report?
Read More>>> https://www.alliedmarketresearch.com/medical-devices-regulatory-affairs-market-A13369
Table of Content
Chapter One: Industry Overview
Chapter Two: Major Segmentation (Classification, Application and etc.) Analysis
Chapter Three: Production Market Analysis
Chapter Four: Sales Market Analysis
Chapter Five: Consumption Market Analysis
Chapter Six: Production, Sales and Consumption Market Comparison Analysis
Chapter Seven: Major Manufacturers Production and Sales Market Comparison Analysis
Chapter Eight: Competition Analysis by Players
Chapter Nine: Marketing Channel Analysis
Chapter Ten: New Project Investment Feasibility Analysis
Chapter Eleven: Manufacturing Cost Analysis
Chapter Twelve: Industrial Chain, Sourcing Strategy and Downstream Buyers
Thank you for reading the article, Regional reports like North America, Europe, Asia-Pacific, LAMEA are also available.
Contact Us:
David Correa
Portland, OR, United States
USA/Canada (Toll Free): +1-800-792-5285, +1-503-894-6022,
UK: +44-845-528-1300
Hong Kong: +852-301-84916
India (Pune): +91-20-66346060
Fax: +1(855)550-5975
help@alliedmarketresearch.com
Web: https://www.alliedmarketresearch.com
About Us:
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Portland, Oregon. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Medical Devices Regulatory Affairs Market Update- See How Industry Players are Preparing Against U.S. Breakdown Depression here
News-ID: 2777219 • Views: …
More Releases from Allied Market Research
![[CAGR of 5.4%] Carbon Molecular Sieves Market Size, 2026 | Growing Demand and Business Outlook by 2031](https://cdn.open-pr.com/L/2/L227367535_g.jpg)
[CAGR of 5.4%] Carbon Molecular Sieves Market Size, 2026 | Growing Demand and Bu …
The global carbon molecular sieves market was estimated at $0.9 billion in 2021 and is expected to hit $1.4 billion by 2031, registering a CAGR of 5.4% from 2022 to 2031.
According to the report published by Allied Market Research, The report provides a detailed analysis of the top investment pockets, top winning strategies, drivers & opportunities, market size & estimations, competitive landscape, and evolving market trends. The market study…
![Thermal Interface Material Market [2026-2031], Rapidly Growing Industry at a CAGR of 8.8%](https://cdn.open-pr.com/L/2/L227287363_g.jpg)
Thermal Interface Material Market [2026-2031], Rapidly Growing Industry at a CAG …
According to the report published by Allied Market Research, the global thermal interface material market was estimated at $4.7 billion in 2021, and is projected to reach $10.8 billion by 2031, growing at a CAGR of 8.8% from 2022 to 2031. The report provides a detailed analysis of the top investment pockets, top winning strategies, drivers & opportunities, market size & estimations, competitive landscape, and evolving market trends. The market…
![[CAGR of 4.1%] Malic Acid Market Size Prospects 2026: Trends and Growth Analysis, Forecast to 2031](https://cdn.open-pr.com/L/2/L227735373_g.jpg)
[CAGR of 4.1%] Malic Acid Market Size Prospects 2026: Trends and Growth Analysis …
According to the report published by Allied Market Research, the global malic acid market garnered $187.6 million in 2021, and is estimated to generate $279.8 million by 2031, manifesting a CAGR of 4.1% from 2022 to 2031. The report provides an extensive analysis of changing market dynamics, major segments, value chain, competitive scenario, and regional landscape. This research offers a valuable guidance to leading players, investors, shareholders, and startups in…
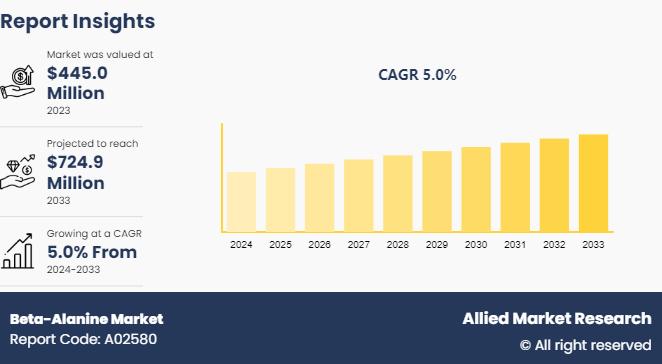
Beta-Alanine Market is Anticipated to Generate USD 724.9 Million by 2033 | AMR
Allied Market Research recently published a report titled, "Global Beta-Alanine Market - Opportunity Analysis and Industry Forecast, 2020-2030". According to the report, the recent technological advancements and launch of new products have a significant influence on the growth. The report includes a detailed analysis of the market trends, major driving factors, prime market players, and top investment pockets. It is vital for new market entrants, stakeholders, and shareholders to make…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
