Press release
Clinical Trials Market Growth Opportunities Investment Analysis Report 2020-2030
Success of Clinical Trials for COVID-19 Vaccines Boosts Market GrowthThe coronavirus pandemic has brought research labs and healthcare institutions under great scrutiny for accelerating the clinical trials for COVID-19 vaccines. As such, the success in these clinical trials has led to global recognition of India for supplying several lack of free doses to Brazil, Bangladesh, Algeria, and South Africa. Countries such as Sri Lanka are following suit whilst creating incremental opportunities for stakeholders in the clinical trials market.
The Food & Drug Administration (FDA) is creating awareness about Coronavirus Treatment Acceleration Program (CTAP) in order to bring economies to normal. Companies in the clinical trials market are taking advantage of this program to make new treatments available to patients as quickly as possible.
Report Overview: https://www.transparencymarketresearch.com/clinical-trials-market.html
Clinical Trial Recruitment Companies Essential for Matching Patients with Right Trial
The clinical trials market is estimated to cross US$ 83.5 Bn by the end of 2030. However, finding the right patients is one of the most important pieces of the puzzle with respect to conducting clinical trials. Hence, stakeholders are working with experts at clinical trial recruitment companies to match patients with the right trials.
Slow recruitment is another challenge faced by companies in the clinical trials market. Hence, companies are becoming aware about scrutinizing existing patient data to anticipate potential recruitment challenges in the long run. Clinical trial innovations are anticipated to bolster participation from volunteers.
FDA Guidelines Pave Way for Innovations for At-home, At-clinic Clinical Trials
Companies in the clinical trials market are following guidelines of the FDA to advance in processes. They are referring to FDA guidance about Severely Debilitating or Life-Threatening Hematologic Disorders (SDLTHDs) in patients. Companies are increasing efforts to fill in the gap between scientific and technical complexities of clinical trials. Thus, companies are investing in improving the academia and are developing new incentives that reward collaboration from volunteers.
Companies in the clinical trials market are taking additional efforts to develop networks that enable procedures at a patient's home or at their private doctor's clinic. This has led to innovations in sensor devices, patient reported outcomes on their computers, and flash pictures of their lesions from their cellphones that contribute toward processes.
Coronavirus Pandemic Pushes Sponsors, Patients to Adopt Virtual Clinical Trials
Compliance with protocols is one of the key trends followed by stakeholders in the clinical trials market. Clinical trials supervised by Principal Investigators are gaining prominence in the market landscape. Continued positive cases for coronavirus have increased relevancy and role of stakeholders in the clinical trials market. This shift has pushed clinical trials to go virtual.
Request Sample: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=78053
Stakeholders in the clinical trials market are teaming up with virtual clinical trial experts to capitalize on business opportunities during the ongoing pandemic. Even regulatory authorities have released guidelines to assist sponsors and patients via telemedicine and virtual trial tools to create viable solutions for current operational problems.
Digital Health Innovations Give Impetus to IoMT for Enhancing Clinical Development Programs
The proliferation of digital innovations with the help of wearables and sensors is translating into value grab opportunities for companies in the clinical trials market. ICON plc- a clinical research organization company is researching how digital endpoints, including digital biomarkers can improve trial outcomes. This has led to the adoption of digital health technologies that enable appropriate device selection and data strategies.
Digital health innovations hold promising potentials to better manage chronic diseases and improve patient access to healthcare services. Stakeholders and sponsors are taking efforts to improve adherence to medications and prevent its complications in patients. The Internet of Medical Things (IoMT) has the potential to enhance clinical development programs involving med-tech and pharmaceutical companies.
Clinical Trials Market: Overview
According to Transparency Market Research's latest report on the global clinical trials market for the historical period 2018-2019 and forecast period 2020-2030, high prevalence and increase in incidence rate of chronic diseases and rise in R&D activities in biotechnology & pharmaceuticals industries are projected to drive the global clinical trials market during the forecast period
According to the report, the global clinical trials market was valued over US$ 46.7 Bn in 2019 and is anticipated to expand at a CAGR of 5.4% from 2020 to 2030
Enquiry Before Buying: https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=78053
High Prevalence and Increase in Incidence Rate of
Chronic Diseases to Drive Demand for Clinical Trials: Key Drivers
Chronic diseases such as chronic respiratory diseases (CRD), diabetes, chronic kidney diseases (CKD), cancer, cardiac stroke, and neurological disorders are the leading causes of disability and mortality across the globe
The emergence and outbreak of various infectious and chronic diseases have created challenges and new opportunities for researchers to develop new diagnostic tools, tests, drugs, and vaccines for early diagnosis, prevention, and cure of such diseases
Hepatitis and HIV are the other major infectious diseases. According to the World Health Organization (WHO), as of October 2017, around 71 million people across the world were estimated to have hepatitis C infection. The WHO also stated that 36.7 million people were affected with HIV across the globe.
An article published by Columbia, Mailman School of Public Health on infectious disease epidemiology stated that infectious diseases continue to have a substantial impact on the health of communities across the world. Various seasonal outbreaks of diseases in certain parts of the world, global epidemics, the threat of resistant bacteria, and the challenge of emerging and newly identified pathogens increase the need of new methods to detect such pathogens, to understand their pathogenesis, and to devise effective interventions for their prevention and control.
These factors have led to an increase in demand for clinical trials, and are expected to drive the clinical trials market from 2020 to 2030
Rise in R&D Activities in Biotechnology and Pharmaceuticals Industries Boosts Market Growth
Clinical research organizations, diagnostic laboratories, and biotechnology players are engaged in the development of newer diagnostic tests to address the unmet needs in the healthcare industry. The life science industry's R&D spending is driven primarily by the mass and research intensity of the biopharmaceutical sector, which accounts for nearly 85% of all expenditure.
Additionally, laboratories strive to improve equipment cost and performance. UHPLC and ultra-fast mass spectrometers are more reliable, faster, and more sensitive as a result of research initiatives by major players.
Acceleration in biopharmaceutical R&D innovation, buoyed by several contributing factors such as precision medicine getting into gear in rare diseases, cancer, and autoimmune diseases; immunotherapy, expansion of therapeutic modalities exploiting natural and synthetic biology innovation, and expedited regulatory pathways boost the growth of the clinical trials market
Request Customization: https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=78053
Patient Recruitment and Retention to Hamper Global Market
Shift in focus toward genetic and rare diseases has made recruitment of relevant patient population a challenging task. Moreover, lack of awareness among patients of clinical trials presents a challenging environment for CROs.
Lack of appropriate patient recruitment could have an impact on the scientific and financial viability. According to the Tufts Center for the Study of Drug Development, in 2013, 11% of the sites failed to recruit even a single patient for clinical trial.
Other factors responsible for poor patient recruitment are patient's fear of side effects, illiteracy, language barrier for region-specific clinical trials, and recording of the consent process
Factors affecting the retention of patients for clinical trials include serious adverse events, fear of complex medical procedures, poor compliance of protocol, lack of dedication toward patient safety, and lack of support from family. This significantly affects the advancement of clinical trial process and product development.
Clinical Trials Market: Competition Landscape
This report profiles major players in the global clinical trials market based on various attributes such as company overview, financial overview, product portfolio, business strategies, and recent developments
Leading players operating in the global clinical trials market are
Laboratory Corporation of America Holdings
IQVIA, Inc.
Syneos Health
Parexel International Corporation
PRA Health Sciences, Inc.
PPD, Inc.
Icon plc
Charles River Laboratories, Inc.
WuXi AppTec
Medpace Holdings, Inc.
More Trending Reports of Transparency Market Research:
Controlled Substance Market: https://www.transparencymarketresearch.com/us-controlled-substance-market.html
Tree Nut Allergy Market: https://www.transparencymarketresearch.com/us-tree-nut-allergy-market.html
Cannabinoids Market: https://www.transparencymarketresearch.com/cannabinoids-market.html
Dermal Fillers Market: https://www.transparencymarketresearch.com/dermal-fillers-market.html
Interspinous Spacers Market: https://www.transparencymarketresearch.com/interspinous-spacer-market.html
Orthopedic Fracture Repairing Implants for
Osteoporosis Market: https://www.transparencymarketresearch.com/orthopedic-fracture-repairing-implants-for-osteoporosis-market.html
Collagen Dressings Market: https://www.transparencymarketresearch.com/collagen-dressings-market.html
Healthcare Biometrics for Children Market: https://www.transparencymarketresearch.com/healthcare-biometrics-for-children-market.html
Contact
Rohit Bhisey
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Blog: https://tmrblog.com
Email: sales@transparencymarketresearch.com
About Transparency Market Research
Transparency Market Research registered at Wilmington, Delaware, United States, is a global market research company providing custom research and consulting services. TMR provides in-depth insights into factors governing demand in the market. It divulges opportunities across various segments based on Source, Application, Sales Channel, and End-Use that will favor growth in the market over the next 9 years.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
For More Research Insights on Leading Industries, Visit Our YouTube Channel and hit subscribe for Future Update - https://www.youtube.com/channel/UC8e-z-g23-TdDMuODiL8BKQ
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trials Market Growth Opportunities Investment Analysis Report 2020-2030 here
News-ID: 2705993 • Views: …
More Releases from Transparency Market Research
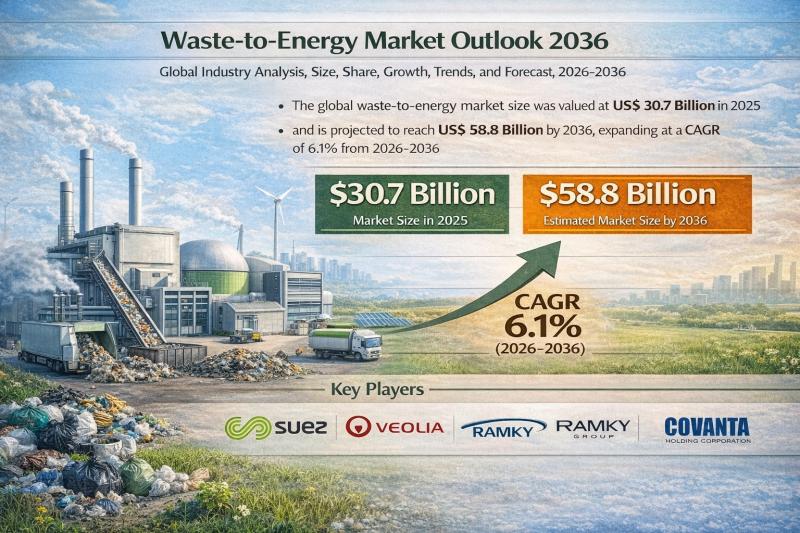
Waste-to-Energy Market to Reach US$ 58.8 Billion by 2036 at 6.1% CAGR | Transpar …
The global waste-to-energy (WtE) market is entering a dynamic growth phase as governments and industries intensify efforts to transition toward sustainable waste management and renewable power generation. Waste-to-energy refers to the process of converting municipal solid waste (MSW), agricultural waste, and other refuse into usable forms of energy such as electricity, heat, and fuel through technologies including incineration, gasification, pyrolysis, and anaerobic digestion.
As environmental pressures mount and landfill capacity shrinks,…
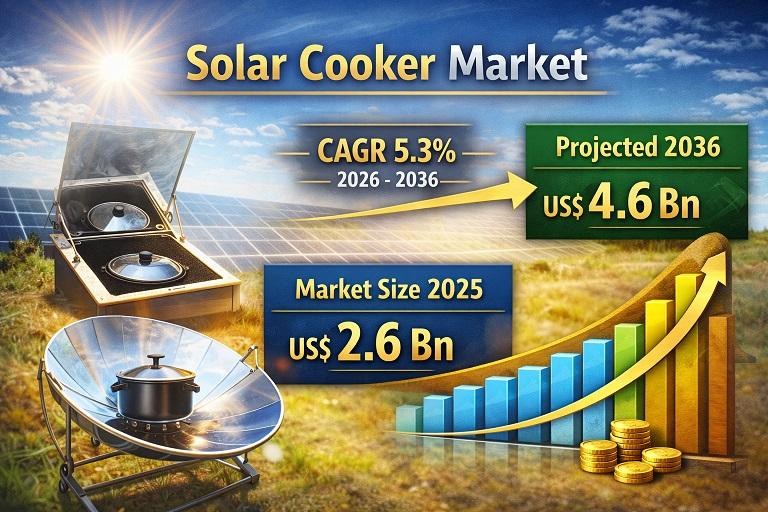
Solar Cooker Market to Reach USD 4.6 Billion by 2036, Driven by Rising Demand fo …
The Solar Cooker Market is witnessing steady expansion as global demand for sustainable and clean cooking solutions accelerates. Solar cookers, which utilize sunlight as a renewable heat source for food preparation, are gaining recognition as eco-friendly alternatives to conventional cooking methods that rely on fossil fuels, firewood, and electricity. Rising environmental awareness, increasing fuel costs, and government initiatives promoting renewable energy adoption are key factors supporting market growth worldwide.
The Solar…
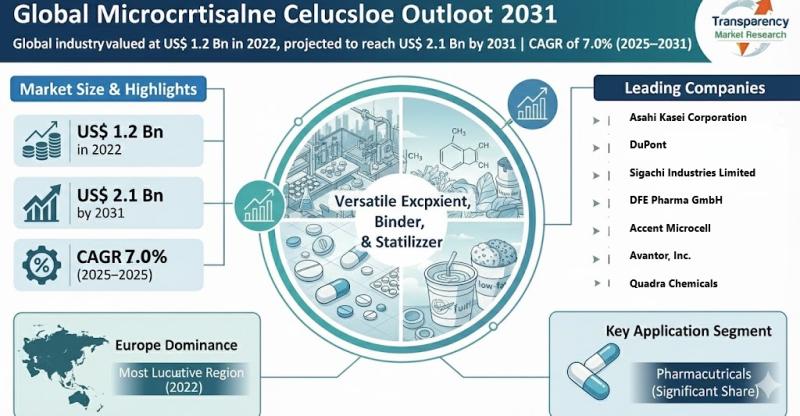
Microcrystalline Cellulose Market to Reach US$ 2.1 Bn by 2031, Driven by Pharmac …
The global microcrystalline cellulose (MCC) market was valued at US$ 1.2 Bn in 2022 and is projected to reach US$ 2.1 Bn by the end of 2031, expanding at a CAGR of 7.0% from 2023 to 2031. The market is expected to witness robust growth driven by increasing demand from the pharmaceutical and food & beverage industries, expanding applications in personal care products, and rising consumer preference for clean-label and…
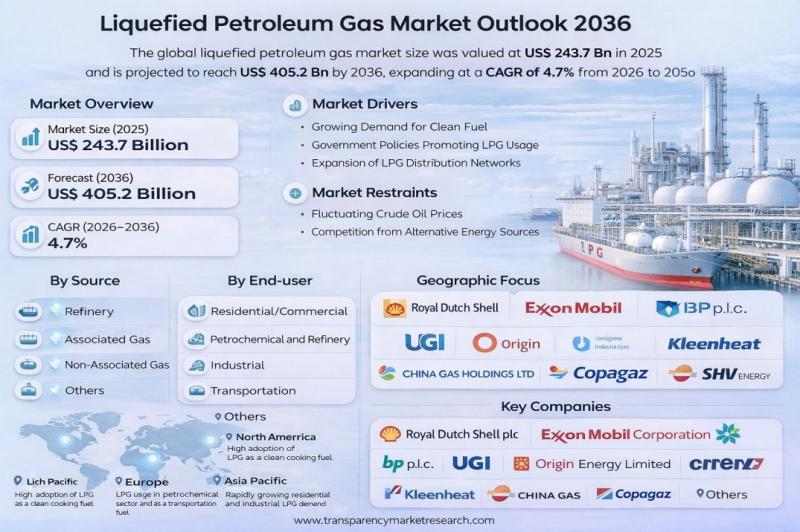
Liquefied Petroleum Gas Market to Reach US$ 405.2 Billion by 2036, Driven by Shi …
The global liquefied petroleum gas (LPG) market was valued at US$ 243.7 Billion in 2025 and is projected to reach US$ 405.2 Billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.7% from 2026 to 2036. Market growth is primarily driven by the accelerating global transition toward cleaner and lower-carbon fuels, coupled with rising residential and commercial energy demand in emerging economies.
Access key findings and insights from…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
