Press release
eClinical Solutions and Software Market Will Generate Booming Growth Opportunities to 2032
At a CAGR of 11.7 percent from 2022-2032, the global eClinical solutions and software market is expected to reach USD 15.4 billion by 2026, up from USD 8.3 billion in 2021. Some of the major driving factors for the global demand for eClinical solutions and software include rising operational costs and compliance standards associated with clinical scientific studies, financial assistance to support clinical trials, and extensive R&D spending on drug development by pharma-biotech companies. One of the important factors driving the eClinical solutions and software market size is biopharma and pharma businesses increasing their research and development operations. Aside from that, the industry is expected to benefit from the increasing use of software solutions in clinical trials. The COVID-19 pandemic has boosted market adoption, as corporations have been investing in improved healthcare IT systems to aid in the fight against the pandemic.As the volume of data created during clinical development processes grows, so does the demand for analyzing and managing clinical data. As a result, the use of eClinical solutions in clinical trials is becoming more common. Furthermore, eClinical technologies improve site performance, increase clinical trial efficiency, and reduce costs by removing redundant data entry. The eClinical solutions and software market is predicted to grow as a result of the aforementioned causes. In addition, the rapid use of eClinical solutions such as RTSM in order to enroll and randomize patients, as well as effective trial medication supply management, is expected to boost eClinical solutions revenue. During the research lifecycle, eClinical solutions assist researchers in organizing, standardizing, and managing their clinical research data and information. Several integrated eClinical solutions (such as CTMS and CDMS) offer clinical researchers end-to-end solutions for all clinical trial operations. However, these programs are costly and premium-priced. eClinical systems cost around USD 2 million to install and maintain, with additional expenditure for technical support for cloud-based solutions. Advanced eClinical software systems' high costs are projected to limit their ideal acceptance among price-sensitive and small-sized end customers, such as pharma-biotech businesses, independent researchers, and CROs.
As per the global market study on eClinical solution software, due to the presence of a large and genetically diverse population, high illness prevalence, and low-cost outsourced services, Asian countries are emerging as significant outsourcing destinations for clinical trials. Clinical trials are contracted to CROs in China and India in large numbers. The rise in the number of databases shared among research institutions, CROs, collaborators, and software businesses elevates the danger of data leakage. The use of electronic tools for patient databases raises privacy concerns concerning patient records, posing a significant problem for pharmaceutical companies in terms of adhering to privacy policies when utilizing eClinical software solutions.
Request Sample PDF Brochure:
https://www.futuremarketinsights.com/reports/sample/rep-gb-14416
Key Takeaways
• In 2021, the electronic data capture (EDC) segment had a revenue share of 21.9 percent and was expected to grow at a healthy rate during the forecast period.
• The eClinical solutions and software market is divided into four phases based on the stage of the clinical trial: phase I, phase II, phase III, and phase IV. In 2021, the Phase III category generated USD 3,383.2 million in market revenue, and similar trends are predicted from 2021 to 2027. As a growing number of medications make it to phase III, segmental growth will accelerate, propelling the demand for eClinical solutions and software forward.
• During the analysis period, the contract research organization (CRO) market is predicted to grow at a stable CAGR of roughly 12.5 percent. The increasing number of CROs on the market accounts for the high segmental growth rate.
• In 2021, the eClinical solutions and software market for North American eClinical solutions topped USD 3,672.5 million, and it is expected to continue to rise steadily in the future years. The prominent presence of major pharmaceutical and medical device businesses is credited with the high growth rate. Furthermore, the availability of advanced infrastructure is likely to enhance the corporate environment.
Competitive Landscape
Companies functioning in the eClinical solutions and software market are anticipated to benefit from the adoption of business strategies because it will allow them to grow.
Oracle Corporation, PAREXEL International, Medidata, Clario, Merge Healthcare (IBM), Datatrak Int, and ALTEN Group (Axial) are among the leading market leaders in eClinical solutions sector. The most commonly used business strategies to maintain market position are new service launches, partnerships, and collaboration.
Key Developments:
• With a new edition of SmartSignals eConsent in April 2021, Signant Health increased electronic informed consent services and boosted capabilities. To fulfill the study needs of teams, SmartSignals eConsent solutions are available in three categories of functionality: silver, gold, and platinum. This method has improved the company's existing product offering, resulting in revenue from its sale.
• In January 2021, Parexel International Corporation teamed up with Signify Health to provide innovative technologies to improve clinical trial access and inclusion. This collaboration increased patient access to clinical trials by bringing studies to their homes and also identified key social determinants of health (SDoH) to help patients, caregivers, and resources connect.
Key Market Segments
By Solution:
• Randomization & Trial Management (RTSM)
• Clinical Data Management System (CDSM)
• Clinical Trial Management System (CLMS)
• Electronic Clinical Outcome Assessment (eCOA)
• Electronic Trial Master File (eTMF)
• Electronic Data Capture
• Others
By Delivery Mode:
• Licensed Enterprise (on-premise) Solution
• Cloud-based (SAAS) Solution
• Web-hosted (on-demand) Solution
By Clinical Trial:
• Phase I
• Phase II
• Phase III
• Phase IV
By End Use:
• contract Research Organization
• Medical Device Companies
• Pharma/biotech Companies
• Hospitals & Clinics
• Others
By Region:
• North America
• Latin America
• Europe
• Asia Pacific
• Middle East and Africa
Ask for Customization:
https://www.futuremarketinsights.com/customization-available/rep-gb-14416
Table of Content
1. Executive Summary
1.1. Global Market Outlook
1.2. Demand-side Trends
1.3. Supply-side Trends
1.4. Technology Roadmap Analysis
1.5. Analysis and Recommendations
2. Market Overview
2.1. Market Coverage / Taxonomy
2.2. Market Definition / Scope / Limitations
3. Market Background
3.1. Market Dynamics
3.1.1. Drivers
3.1.2. Restraints
3.1.3. Opportunity
3.1.4. Trends
3.2. Scenario Forecast
3.2.1. Demand in Optimistic Scenario
3.2.2. Demand in Likely Scenario
3.2.3. Demand in Conservative Scenario
3.3. Opportunity Map Analysis
3.4. Product Life Cycle Analysis
3.5. Investment Feasibility Matrix
3.6. PESTLE and Porter's Analysis
3.7. Regulatory Landscape
3.7.1. By Key Regions
3.7.2. By Key Countries
3.8. Regional Parent Market Outlook
4. Global eClinical Solutions and Software Market Analysis 2017-2021 and Forecast, 2022-2032
4.1. Historical Market Size Value (US$ Mn) Analysis, 2017-2021
4.2. Current and Future Market Size Value (US$ Mn) Projections, 2022-2032
4.2.1. Y-o-Y Growth Trend Analysis
4.2.2. Absolute $ Opportunity Analysis
5. Global eClinical Solutions and Software Market Analysis 2017-2021 and Forecast 2022-2032, By Solution
5.1. Introduction / Key Findings
5.2. Historical Market Size Value (US$ Mn) Analysis By Solution, 2017-2021
5.3. Current and Future Market Size Value (US$ Mn) Analysis and Forecast By Solution, 2022-2032
5.3.1. Randomization & Trial Supply Management (RTSM)
5.3.2. Clinical Data Management System (CDMS)
5.3.3. Clinical Trial Management System (CLMS)
5.3.4. Electronic Clinical Outcome Assessment (eCOA)
5.3.5. Electronic Trial Master File (eTMF)
5.3.6. Electronic Data Capture
5.3.7. Others
5.4. Y-o-Y Growth Trend Analysis By Solution, 2017-2021
5.5. Absolute $ Opportunity Analysis By Solution, 2022-2032
Inquire Before Buying This Research Report:
https://www.futuremarketinsights.com/ask-question/rep-gb-14416
Contact:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
LinkedIn| Twitter| Blogs
About Us
Future Market Insights (ESOMAR certified market research organization and a member of Greater New York Chamber of Commerce) provides in-depth insights into governing factors elevating the demand in the market. It discloses opportunities that will favor the market growth in various segments on the basis of Source, Application, Sales Channel and End Use over the next 10-years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release eClinical Solutions and Software Market Will Generate Booming Growth Opportunities to 2032 here
News-ID: 2701215 • Views: …
More Releases from Future Market Insights
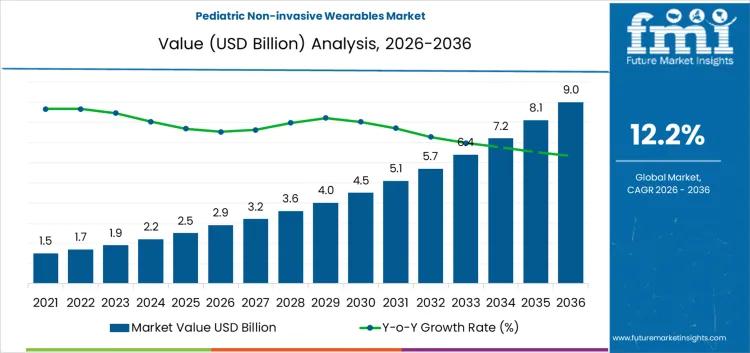
Pediatric Non-invasive Wearables Market Growth, Trends, Company Profiles, Market …
Future Market Insights (FMI), a leading market intelligence and consulting firm, today released its latest report on the pediatric non-invasive wearables market. The comprehensive study examines the global landscape of devices designed for continuous monitoring of children's vital signs, projecting significant expansion from USD 3.2 billion in 2026 to USD 9 billion by 2036. This growth, at a compound annual growth rate (CAGR) of 12.2%, underscores the increasing role of…
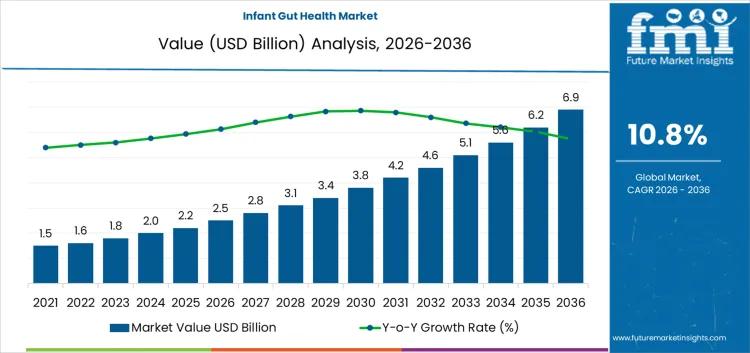
Infant Gut Health Market Growth, Analysis, Trends, Recent Developments and Forec …
Future Market Insights (FMI) has released a detailed report on the infant gut health market, projecting steady growth driven by increasing recognition of the gut microbiome's influence on early childhood development. The market, valued at USD 2.8 billion in 2026, is expected to reach USD 6.9 billion by 2036, expanding at a compound annual growth rate (CAGR) of 10.8%. This forecast underscores the sector's response to prevalent digestive challenges in…
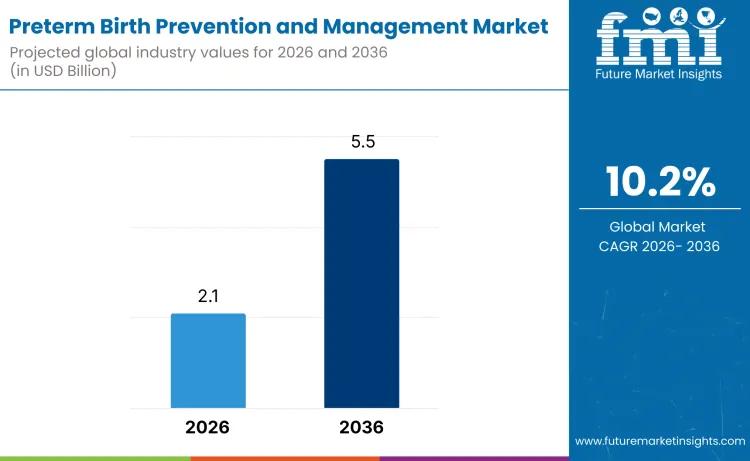
Preterm Birth Prevention and Management Market Growth, Key Players, SWOT, Revenu …
Future Market Insights (FMI), a leading provider of market intelligence and consulting services, today released a comprehensive report on the global preterm birth prevention and management market. The analysis forecasts significant growth from USD 2.1 billion in 2026 to USD 5.5 billion by 2036, registering a compound annual growth rate (CAGR) of 10.2%. This expansion underscores the increasing emphasis on maternal and neonatal health worldwide, addressing a critical issue where…
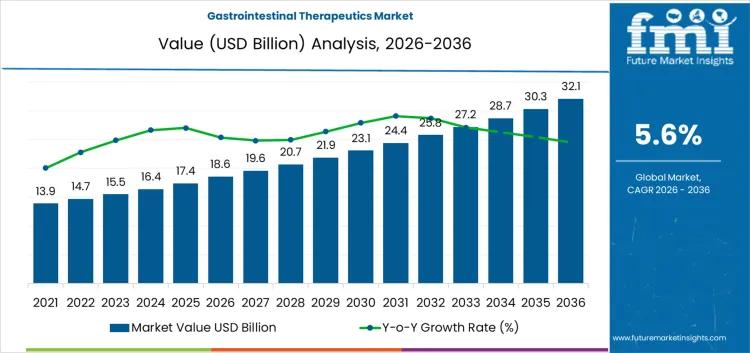
Gastrointestinal Therapeutics Market Growth, Opportunities, Trends, Factors, Rev …
Future Market Insights (FMI) today published a detailed analysis of the global gastrointestinal therapeutics market, forecasting steady expansion driven by rising incidences of chronic digestive disorders. The market, valued at USD 18.6 billion in 2026, is expected to reach USD 32.1 billion by 2036, advancing at a compound annual growth rate (CAGR) of 5.6%. This growth reflects increasing demand for treatments addressing conditions such as inflammatory bowel disease (IBD), irritable…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
