Press release
Rapid Diagnostics Market Worth $18.20 Billion by 2029
According to a new market research report titled "Rapid Diagnostics Market by Product (Kits [OTC, Professional], Readers), Platform (Lateral Flow, Serological, PCR), Application (Blood Glucose, Infectious Diseases, Pregnancy, Drugs of Abuse), End User (Hospitals, Diagnostic Labs, Home Care) - Global Forecasts to 2029", published by Meticulous Research®, the rapid diagnostics market is expected to grow at a CAGR of 8.9% from 2022-2029 to reach $18.20 billion by 2029.Download Free Sample Report Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=5240
A rapid diagnostic test (RDT) is a medical diagnostic test intended to provide diagnostic results efficiently and immediately (within 1 hour). RDT kits are widely used in hospitals, clinics, and diagnostic laboratories for the qualitative and quantitative detection of specific antigens, antibodies, and genetic material or proteins associated with a specific health condition or disease.
The growth of the overall RDT market is driven by key factors, such as the growing demand for POC diagnostics, the rising prevalence of chronic diseases coupled with the increasing aging population, the growing necessity for rapid decision making in emergency departments, and emerging technological innovations.
Impact of COVID-19 on the Rapid Diagnostics Market
The COVID-19 pandemic positively impacted the rapid diagnostics market in terms of revenue; however, the pandemic negatively impacted the sales of routine testing kits used mainly to screen cancer, blood glucose, cardiac markers, lipid profile, cholesterol, and other infectious diseases. The COVID-19 pandemic led to a reduction in the number of overall diagnostic testing conducted globally, which translated further into reduced instrument installations and reduced sales of reagents and consumables, but the loss of sales occurred in routine testing kits was compensated by high sales of COVID-19-related tests. The loss of sales of routine testing kits was mainly affected due to factors such as:
The restrictions on local travel and public gatherings discouraged patients from visiting physicians, health practices, and hospitals. These restrictions largely affected elderly patients.
As many hospitals diverted their resources towards treatment and management of COVID-19 infections, it was observed that hospitals and health practices experienced a certain level of operational disruptions leading to delays or cancellations of patient visits, especially for non-critical procedures.
Companies operating in the market reported an increase in forward purchases in the first quarter of 2020 as distributors stocked up products in anticipation of lockdowns, which resulted in supply chain disruptions. This led to a higher level of sales prior to restrictions being imposed, followed by a lower level of sales during the second quarter of 2020.
To tackle the pandemic, governments worldwide depended on rapid diagnostic kits to diagnose COVID-19 infections. Also, various governments took several initiatives to support the testing needs of their respective countries. Some of them are listed below:
In May 2021, the Indian Council of Medical Research (ICMR) approved the self-use Rapid Antigen Test for COVID-19 developed by Mylab Discovery Solutions Pvt. Ltd. (India), designed for home testing to screen symptomatic individuals and people who came in contact with infected patients.
In April 2021, the Japanese Government launched a new COVID-19 negative proof system known as, TeCOT (COVID-19 Testing Center for Overseas Travelers) on smartphones, via which a certificate was provided to individuals whose PCR test for COVID-19 was negative. The main aim of this application was to support the smooth movement of people traveling overseas for work.
In December 2020, the French Government launched a mass COVID-19 screening campaign in the French cities of Le Havre, on the Normandy coast, and Charleville-Mézières to control the spread of the disease.
In November 2020, Chinese authorities ordered mass testing of the entire city of Manzhouli after some suspicious cases of COVID-19 infections were identified in the city.
In October 2020, the Italian Health Ministry announced rapid COVID-19 diagnostics tests to be conducted throughout the country to control the rising incidences of COVID-19 infections.
In June 2020, to promote the number of COVID-19 tests conducted across the country, the Indian Council of Medical Research (ICMR) encouraged other manufacturers/developers of rapid diagnostics kits with antigen detection assays to come forward for validation.
It was observed that during the COVID-19 pandemic, many diagnostics products were launched to fulfill the demand for COVID-19 infection diagnosis. Also, companies operating in the market started to hold web meetings and webinars to interact with their customers and shareholders and provide product descriptions, customer demonstrations, and applications training.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business: https://www.meticulousresearch.com/speak-to-analyst/cp_id=5240
Thus, the COVID-19 pandemic negatively impacted the sales of routine testing kits globally, but high sales of the COVID-19-related tests compensated for the loss of sales in routine testing kits.
Rapid Diagnostics Market: Future Outlook
The overall rapid diagnostics market is segmented based on product (kits and test readers/analyzers), platform (immunoassays, molecular detection, and other platforms), application (blood glucose testing, cardiac metabolism testing, infectious diseases testing, coagulation testing, pregnancy & fertility testing, fecal occult testing, hematology testing, tumor/cancer markers testing, drugs of abuse testing, urinalysis, and cholesterol testing), end user (hospitals & clinics, diagnostic laboratories, home care/self testing, and other end users) and geography (North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa). The study also evaluates industry competitors and analyzes their market shares at the global and regional levels.
Based on product type, the kits segment is estimated to account for the largest share of the overall rapid diagnostics market in 2022. The large share of this segment is attributed to the rising adoption and repetitive kits usage. In addition, the growing portfolio of disease-specific kits for the early diagnosis of chronic diseases and the increasing technological advancements are other factors supporting the growth of this segment. However, the test readers/analyzers segment is expected to witness the fastest growth rate during the forecast period. The high growth rate of this segment is attributed to the capabilities of these devices to simultaneously read multiple tests in one scan and scale large volumes. In addition, healthcare facilities adopt analyzers for quick analysis compared to centralized lab analyzers due to their features, such as compact size, portability, and easy accessibility.
Based on platform, the immunoassays segment is estimated to account for the largest share of the overall rapid diagnostics market in 2022. The large share of this segment is attributed to the capability of immunoassay kits to provide fast and accurate results with high sensitivity in point-of-care diagnostics. Countries worldwide require rapid and accurate SARS-CoV-19 testing kits to tackle the COVID-19 pandemic. Thus, companies have started developing immunoassay kits that provide results with higher sensitivity, accuracy, and speed. For instance, in May 2021, Stream Bio (U.K.) and Chelsea Technologies (U.K.) formed a joint venture, Brightline Diagnostics (DX), and created a unique platform known as Claritas that couples fluorescent lateral flow test with a highly sensitive handheld reader, delivering significant advantages over existing assays. However, the molecular detection segment is expected to witness the fastest growth rate during the forecast period. The growing need for advanced diagnostic techniques and the launch of innovative technologies are factors attributed to the high growth rate of this segment. In addition, the high demand for rapid PCR diagnostic kits due to the COVID-19 pandemic further boost the growth of this segment. For instance, countries such as Japan and India rely heavily on RT-PCR kits to diagnose and manage COVID-19 infections. Also, numerous countries worldwide have made negative COVID-19 RT-PCR test reports mandatory for international and local travelers.
Based on application, the infectious disease testing segment is estimated to account for the largest share of the rapid diagnostics market in 2022. The large share of this segment is mainly attributed to the rising prevalence of infectious diseases and the growing demand for affordable infectious disease testing in emerging markets. For instance, in the U.S., the number of estimated new hepatitis A virus (HAV) infections increased by nearly three folds in 2017 compared to 2014. However, the tumor/cancer markers testing segment is expected to witness the fastest growth rate during the forecast period. The growing incidences of cancer among the general population is the major factor attributed to the high growth rate of this segment. According to WHO, cancer was the leading cause of death globally in 2020, accounting for approximately 10 million deaths. Furthermore, nearly 70% of deaths out of the total deaths from cancer occur in low- and middle-income countries; therefore, early detection is extremely important to reduce mortality, enhance survival, and save treatment costs. Thus, healthcare workers utilize various rapid diagnostic kits for the early detection of cancer.
Quick Buy - Rapid Diagnostics Market Research Report: https://www.meticulousresearch.com/Checkout/74262428
Based on end user, the hospitals & clinics segment is expected to witness the fastest growth rate in the forecast period. The rising number of hospitals worldwide and the increase in healthcare access & expenditure are attributed to the high growth rate of this segment. For instance, the American Hospital Association has reported that the total number of registered hospitals in the U.S. was estimated to grow by 12% to 6,210 in 2019 from 5,534 hospitals in 2018. Similarly, in India, according to the Health Management Information System Portal (HMIS), as of 2018, there were around 37,725 hospitals, which was an increase of 7.0% from 35,416 hospitals in 2013.
Geographically, North America is estimated to command the largest share of the global rapid diagnostics market in 2022, followed by Asia-Pacific, Europe, Latin America, and the Middle East & Africa. The large market share of this region is mainly attributed to the high number of laboratory tests performed annually in North America, the rising prevalence of chronic diseases coupled with the increasing aging population, and the rising number of inpatient hospitalizations. For instance, the U.S. Department of Health and Human Services reported that the number of patients with HIV is growing with a CAGR of 2.5% in the country. It is assumed that 40% of new HIV infections in the U.S. are transmitted by people living with undiagnosed HIV; this boosts the demand for related rapid testing products throughout the country.
However, Asia-Pacific region is expected to be the fastest-growing regional market due to the growing geriatric population, the rising prevalence of infectious & chronic diseases, and various government initiatives to promote health awareness.
Key companies operating in the global rapid diagnostics market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), Bio-Rad Laboratories, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), ACON Laboratories, Inc. (U.S.), Creative Diagnostics (U.S.), Danaher Corporation (U.S.), Thermo Fisher Scientific Inc. (U.S.), BioMérieux S.A. (France), Alfa Scientific Designs, Inc. (U.S.), BTNX Inc. (Canada), Meridian Bioscience, Inc. (U.S.), and Trinity Biotech plc (Ireland) among others.
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/rapid-diagnostics-market-5240
Contact:
Mr. Khushal Bombe
Meticulous Market Research Inc.
1267 Willis St, Ste 200 Redding,
California, 96001, U.S.
USA: +1-646-781-8004
Europe : +44-203-868-8738
APAC: +91 744-7780008
Email- sales@meticulousresearch.com
Visit Our Website: https://www.meticulousresearch.com/
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
Content Source: https://www.meticulousresearch.com/pressrelease/461/rapid-diagnostics-market-2029
We are the trusted research partners for leading businesses around the world, providing market intelligence focused towards building revenue transformation strategies. Our research is used by Fortune 500 organizations to attain success by scouting next generation revenue opportunities well ahead of their competition.
Through our mixed bag of research offerings - syndicated research, custom research, and analyst engagement - we enable smart decision making to enhance business performance for global organizations.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Rapid Diagnostics Market Worth $18.20 Billion by 2029 here
News-ID: 2647292 • Views: …
More Releases from Meticulous Research®
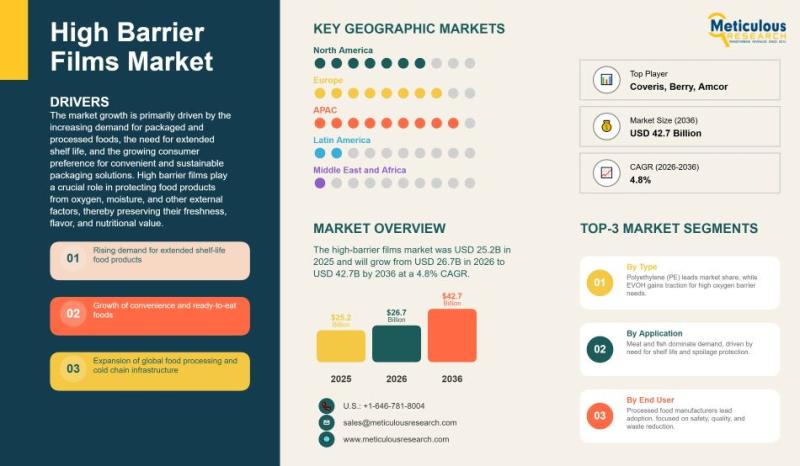
Global High-Barrier Films Market for Food Packaging: Trends, Applications, and G …
The global high-barrier films market for food packaging has experienced notable growth over the past several years, driven by evolving consumer preferences, technological advancements, and increasing demand for packaged food products. In 2025, the market was valued at approximately USD 25.2 billion. High-barrier films, designed to provide superior protection against oxygen, moisture, light, and other environmental factors, are essential for preserving the flavor, texture, and nutritional value of food products.…
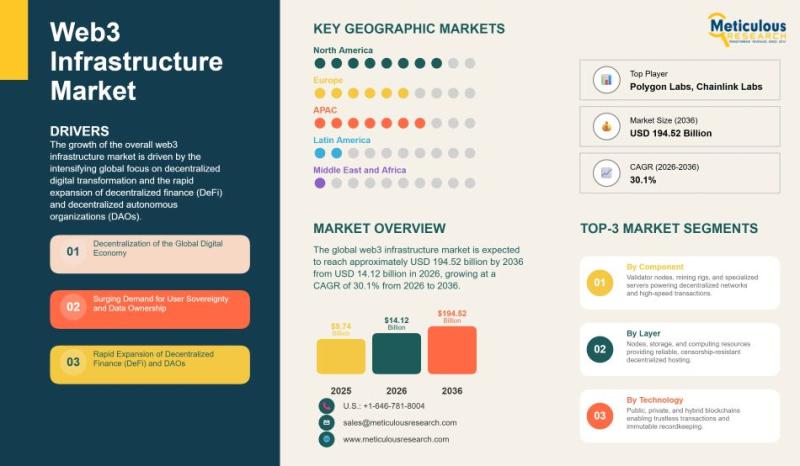
Global Web3 Infrastructure Market Analysis and Forecast (2026-2036)
The global web3 infrastructure market is experiencing rapid growth, driven by a shift toward decentralized digital transformation and the increasing adoption of decentralized finance (DeFi) and decentralized autonomous organizations (DAOs). In 2025, the market was valued at approximately USD 9.74 billion, and it is expected to grow to around USD 14.12 billion in 2026. By 2036, the market is projected to reach USD 194.52 billion, expanding at a compound annual…
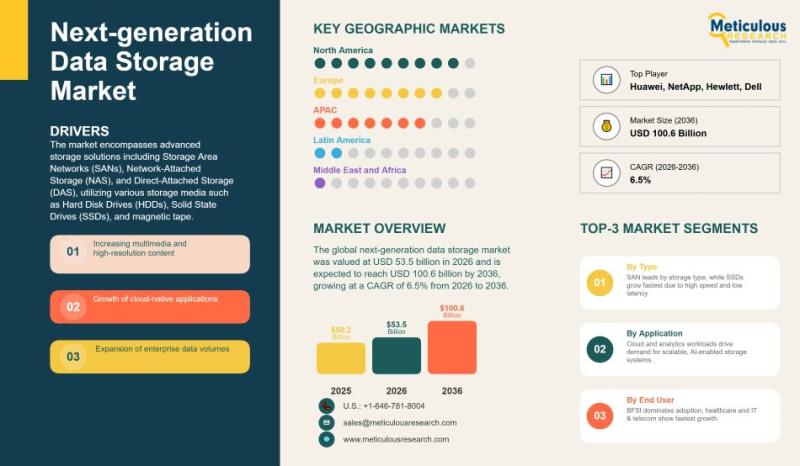
Global Next-Generation Data Storage Market Forecast 2026-2036: Trends, Drivers, …
The global next-generation data storage market is witnessing substantial growth as organizations increasingly grapple with massive volumes of digital information and the need for efficient, scalable storage solutions. In 2026, the market was valued at USD 53.5 billion and is expected to reach USD 100.6 billion by 2036, growing at a compound annual growth rate of 6.5%. This market includes a variety of advanced storage technologies, such as Storage Area…
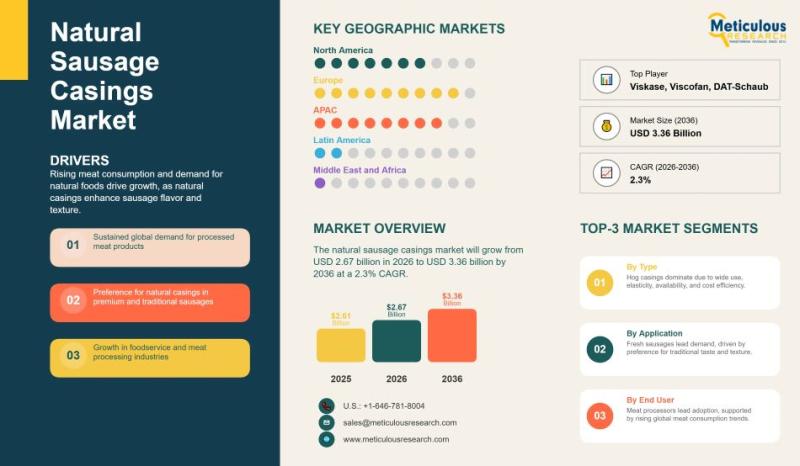
Global Natural Sausage Casings Market 2026-2036: Trends, Growth Drivers, Applica …
The market for natural sausage casings has been growing steadily over the past few years, and it doesn't seem to be slowing down anytime soon. In 2025, it was worth about USD 2.61 billion, and estimates show it could hit USD 3.36 billion by 2036. Even in 2026, the market is expected to be around USD 2.67 billion, growing at a slow but steady pace of roughly 2.3% a year.…
More Releases for Diagnostic
Rubella Diagnostic Testing Market Report 2024 - Rubella Diagnostic Testing Marke …
"The Business Research Company recently released a comprehensive report on the Global Rubella Diagnostic Testing Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive…
Diagnostic Electrocardiograph Market - Elevating Cardiac Care: Diagnostic Electr …
Newark, New Castle, USA: The "Diagnostic Electrocardiograph Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Diagnostic Electrocardiograph Market: https://www.growthplusreports.com/report/diagnostic-electrocardiograph-market/7804
This latest report researches the industry structure, sales, revenue,…
Diagnostic Imaging Market - Empowering Precision Medicine: Harnessing the Potent …
Newark, New Castle, USA: The "Diagnostic Imaging Market" provides a value chain analysis of revenue for the anticipated period from 2021 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Diagnostic Imaging Market: https://www.growthplusreports.com/report/diagnostic-imaging-market/7688
This latest report researches the industry structure, sales, revenue,…
Rubella Diagnostic Testing Market - Advancing Rubella Control: Innovating Diagno …
Newark, New Castle, USA - new report, titled Rubella Diagnostic Testing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Rubella Diagnostic Testing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Rubella Diagnostic Testing market. The report offers an overview of…
Tenet Diagnostic: Reshaping the Diagnostic Industry in India
Tenet Diagnostic was incorporated in 2018 with an aim to establish high-quality diagnostics laboratories across India. The company caters to both B2B and B2C. They have seven different verticals namely: End Customer (B2C), Lab to Lab Test Outsourcing, Corporates and Institutions, large management (Hospitals, Medical Colleges, and other healthcare institutions), Clinical Trials, Home Sample Collection, and Government PPP Projects.
Tenet Diagnostics got its NABL certification in the fastest time and is…
Diagnostic Imaging Services Market 2019 Analyzed by Top Key Players PH3 Healthca …
Big Market Research has added a report, titled, "Diagnostic Imaging Services" The report not only provides a comprehensive analysis of market overview and dynamics for the historical period, 2019-2026, but also offers global and regional forecasts on market value, volume production, and consumption during the future period, 2019-2026. The report also analyzes the key market players, especially the distributors, along with the industrial chain structure. The evolution of market trends…