Press release
Clinical Trials Software Market to Represent Growing Demand, Business Prospects, New Product Launches, and Platforms by 2030| Leading Players Oracle Corporation, IBM , IQVIA , MasterControl Inc.
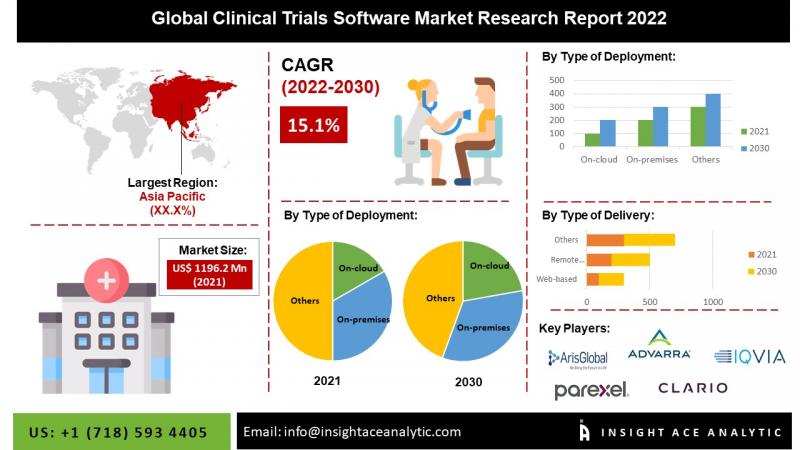
InsightAce Analytic Pvt. Ltd. has announced the publication of a market research report titled "Global Clinical Trials Software Market by (Type of Deployment (On-cloud, On-premises), Type of Delivery (Web-based, Remote Monitoring), Features of software (EDC, eCOA/ePRO, eConsent), End-users (Pharmaceutical & Biotechnology Companies, Contract Research Organizations (CROs), Medical Device Manufacturers, Others)) - Market Outlook and Industry Analysis 2030"According to company's newest research, the global Clinical Trials Software Market is worth US$ 1.20 Billion in 2021 and is predicted to reach US$ 4.13 Billion in 2030, with a promising CAGR of 37 % between 2022 and 2030.
Get Demo Sample copy of Clinical Trials Software Market Report at: https://www.insightaceanalytic.com/request-sample/1195
Clinical trials are an essential tool for determining the efficacy and safety of novel medications, medical devices, and other healthcare interventions. Extended timeframes and high costs involved with bringing new medicines to market have generated a renewed focus on improving clinical trial operational efficiency. As a result, clinical trial sponsors have become more reliant on a range of IT-enabled Clinical Study Management Systems (CTMS) to improve administrative control over trial conduct.
Clinical trial management software, also known as clinical trial software or clinical trial management systems (CTMS), provides a secure, centralized location for collecting, storing, and retrieving data for clinical trials. All trial and study data are centralized in CTMS, which also standardizes and simplifies workflows and tracks and improves site, participant, investigator, and trial processes. A clinical trial management system (CTMS) is a configurable software system used by the biotechnology and pharmaceutical sectors to manage enormous amounts of data associated with clinical trial operations.
It is widely used to maintain and manage clinical trial planning, preparation, performance, and reporting, with a focus on keeping up-to-date contact information for clinical trial participants and tracking milestones and deadlines, such as those used for regulatory approvals or the issuance of progress reports. At the moment, all CTMS offer clinical trial sponsors with improved control during the course of a trial.
Market Dynamics:
Due to the increasing prevalence of chronic diseases, there has been an increase in the number of clinical trials. Clinical trial management services are used by biopharmaceutical companies as a result to speed up overall testing process and improve the quality of the trials. An increase in the number of clinical trials, rapid expansion in healthcare IT, significant R&D expenditures by life science and clinical research organizations, and the acceptance of CTMS solutions are all predicted to drive the widespread use of clinical trial software in the future.
The COVID-19 pandemic has caused global problems, including economic and healthcare crises, as well as spillover effects on global industries. The COVID-19 pandemic has had a negative impact on the market's growth. Clinical trials had to quickly adapt to new techniques of monitoring and caring for trial participants in a world that was rapidly changing. During the pandemic, a range of different clinical trial procedures were used, the most prevalent of which were remote monitoring, video visits, phone visits, eConsent, and EHR. While many of these strategies have been around for a while, the COVID-19 pandemic accelerated their implementation.
For more Customization in this Report, Connect with us at: https://www.insightaceanalytic.com/customisation/1195
Key Developments:
• In April 2022, LifeSphere Clinical software from ArisGlobal has grown significantly in the worldwide footprint following the acquisition of two major universities in the Asia Pacific and Middle East areas. Biopharmaceutical company based in Shanghai, China, has over 15 medications on the market and is working hard to release a COVID-19 treatment for Asia Pacific customers. A new customer in the Middle East, Saudi Arabia, has chosen to use LifeSphere Clinical to support seven therapeutic areas, ranging from rare genetic illnesses to diabetes and breast cancer.
• In Dec 2021, Advarra announced the debut of Advarra Cloud, a new cloud computing service. Advarra customers now have more alternatives for cloud deployment due to this next-generation platform, which provides apps in an easy-to-use, fully managed setting. New capabilities for OnCore clinical trial management, an electronic regulatory management system, comprehensive data analytics and Longboat site training, protocol compliance and patient involvement will be supported immediately by the Advarra Cloud.
• In June 2021, Antidote Technologies Limited has raised $23.2 million to develop its digital patient engagement and clinical trial recruitment services. With this fresh amount of funding, the company will be able to build on its previous commercial success and accelerate its growth. The funds will also be used to improve its clinical trial search engine, create new goods and services (including data insights), and grow its worldwide reach.
• In April 2021, IQVIA Holdings Inc. announced the acquisition of Quest Diagnostics' 40% minority stake in Q2 Solutions. Under a multi-year deal, Quest will continue to be the strategic preferred laboratory provider for Q2 Solutions clients, providing a range of complementary lab testing capabilities to complement Q2 Solutions' core products and extending its industry-leading array of services.
The following key companies are engaged in the Clinical Trials Software market:
Advarra, Antidote Technologies, Inc., ArisGlobal , AssistRx, athenahealth, Inc., Axiom Real-Time Metrics, BioClinica Inc. , BSI Business Systems Integration AG, Calyx , Castor EDC, Chronicles, Clario, Clarivate, ClinCapture, Clincase, Clinical Research, CLIRINX, Cloudbyz, Dacima Software Inc., Datatrak Int. , Florence HC , IBM , Instem , IQVIA , MasterControl Inc., Medidata Solutions Inc, MedNet Solutions Inc., Novatek International, Octalsoft , Openclinica, Oracle Corporation, Parexel International Corp., RealTime Software Solutions LLC, Reify Health, Inc., Signant Health, Statsols, TrialKit , Veeva Systems Inc., WIRB-Copernicus Group, and Other prominent players.
Get Extra Discount on Clinical Trials Software Market Report, if your Company is Listed in Above Key Players List @ https://www.insightaceanalytic.com/enquiry-before-buying/1195
InsightAce Analytic Pvt. Ltd.
Tel.: +1 551 226 6109
Email: info@insightaceanalytic.com
Site Visit: www.insightaceanalytic.com
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trials Software Market to Represent Growing Demand, Business Prospects, New Product Launches, and Platforms by 2030| Leading Players Oracle Corporation, IBM , IQVIA , MasterControl Inc. here
News-ID: 2621689 • Views: …
More Releases from InsightAce Analytic Pvt. Ltd.
High-Temperature Fuel Cells Market Benefits from Technological Advancements and …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global High-Temperature Fuel Cells Market- (By Type (Solid Oxide Fuel Cell, Molten Carbonate Fuel Cell, and Others); By Application (Transportation, Distributed Generation and Others)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global High-Temperature Fuel Cells Market is expected to grow with a CAGR of…

Organic Feminine Care Market to Benefit from Increasing Government Initiatives a …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Organic Feminine Care Market Size, Share & Trends Analysis Report By Product Type (Sanitary Pads, Tampons, Menstrual Cups, Liners and Shields, Others), by Nature (Disposable, Reusable), by Age Group (Upto 18 Years, 19-30 Years, 31-40 Years, 41 Years and Above), by Distribution Channel (Supermarkets and hypermarkets, Pharmacy, Online Stores, Others)- Market Outlook And Industry Analysis…

Intravenous Immunoglobulin Market Key Players Analysis - Biotest AG, Octapharma …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Intravenous Immunoglobulin Market Size, Share & Trends Analysis Report By Application (Hypogammaglobulinemia, CIDP, Congenital AIDS), By Distribution Channel (hospital pharmacies, speciality pharmacies), Region, Market Outlook And Industry Analysis 2031"
The global Intravenous Immunoglobulin market is estimated to reach over USD 21.22 billion by 2031, exhibiting a CAGR of 6.60% during the forecast period.
Get Free Access…

Exascale Computing Market Key Players Analysis- Hewlett Packard Enterprise Compa …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Exascale Computing Market - (By Component (Hardware, Software, Services), By Deployment (On-premises, Cloud-based), By Customer Type (Government & Defense, Healthcare & Biosciences, Financial Services, Research & Academia, Manufacturing & Energy, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Exascale Computing Market is valued at…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…