Press release
The GMP Cell Banking Service Market to Turn Explicit With Innovation
The GMP Cell Banking Service Market is expected to grow on an unabashed note in the next decade. With a worldwide shortage of healthcare staff, the healthcare IT solutions comprising web-based staffing platforms are doing the rounds. The future scenario won't be any different. These platforms would show the gaps, i.e. actual dearth of healthcare personnel like nurse's doctors, technicians, lab workers, and clinicians, which would help the entire healthcare vertical to take measures to bridge the gap between demand and supply. This would be an important development in the healthcare industry going forward.North America is estimated to hold a whopping 45.3% value share in the global GMP cell banking services market by the end of 2017 and will witness an increase of 231 basis points in market share by 2025 over 2017. The North America regional market will create absolute $ opportunity of US$ 23 Mn in 2018 over 2017.
In a new report titled "GMP Cell Banking Services Market: Global Industry Analysis and Forecast, 2017-2025," Persistence Market Research forecasts revenue from the North America market to grow 3.1x by 2025 end as compared to that in 2017. This will directly impact the global GMP cell banking services market, which will likely grow from US$ 346.6 Mn in 2017 to US$ 1,012.4 Mn by the end of 2025, translating into a CAGR of 14.3% during the eight year forecast period 2017 - 2025.
Planning Forward? Access Sample Of GMP Cell Banking Service Market Report! https://www.persistencemarketresearch.com/samples/13735
Company Profiles:
WuXi AppTec
Charles River Laboratories International, Inc.
Eurofins Scientific
Merck KGaA
Lonza Group Ltd
SGS Ltd
ViruSure GmbH
Austrianova
Goodwin Biotechnology Inc.
Paragon Bioservices, Inc.
Strategic industry consolidations and increase in demand for Ready-to-Use (RTU) Bioassay Banks is trending the global GMP cell banking services market
One of the key objectives of the report is to identify the key trends governing the global GMP cell banking services market and present a clear picture of the various forces impacting the market at a micro and macro level. A sustained growth of the market can be attributed to macro-economic drivers such as increased public and private sector funding for disease research, which is expected to bring newer therapies to the market; high costs of clinical development; and various collaborative initiatives to identify therapies for difficult-to-treat illnesses.
On the supply side, an expansion of production facilities by key players to meet growing customer demand besides offering customized solutions based on specific customer needs (this involves strategic tie-ups between biopharmaceutical companies and cell banking service providers) are some of the factors likely to push the global GMP cell banking services market ahead in the coming years. The report also identifies certain demand side drivers boosting the market such as a growing number of biologics in development currently, limited in-house storage and testing capabilities and a rise in the demand for outsourcing and contract manufacturing.
How About Revitalizing The Strategy-Oriented Funnel To Stay Ahead In The GMP Cell Banking Service Market? https://www.persistencemarketresearch.com/methodology/13735
While the above growth factors are indicative of a mature market, Persistence Market Research identifies certain pull factors likely to restrain the market to a certain extent in the coming years. First off, the global GMP cell banking services market is highly fragmented with multiple vendors offering a variety of services - resulting in limited global reach. Besides, complexities in the production and manufacturing processes, a constantly evolving technology landscape, and inconsistent demand for services are likely to restrain revenue growth of the global GMP cell banking services market during the forecast period.
Persistence Market Research predicts a large opportunity for key players in the fast growing Asia Pacific region. It must be noted that there has been a shift of R&D and technology investments in the healthcare industry from North America and Europe to Asia Pacific with developing APAC economies such as India offering increased scope for outsourcing of biotech projects owing to ample skilled and qualified manpower at optimal costs.
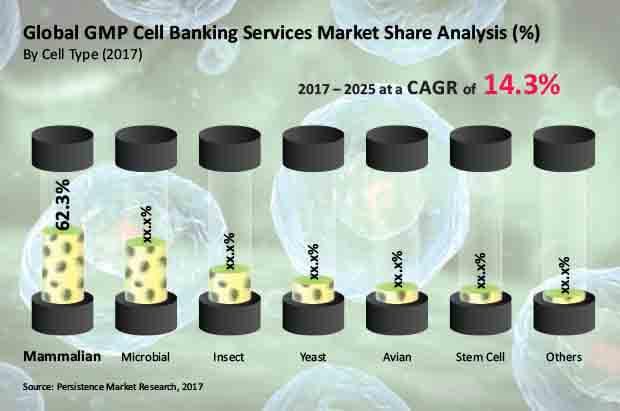
Mammalian cell type segment to retain its pole position throughout the forecast period
Among the cell type segments, the Mammalian segment was the dominant segment in 2016 and is expected to retain its dominance through 2025. From an estimated US$ 216 Mn in 2017 to a market valuation in excess of US$ 680 Mn by the end of the forecast period, the Mammalian segment will register the highest CAGR of 15.6% among the other cell type segments.
The Microbial segment is the second most attractive cell type segment in the global GMP cell banking services market in terms of revenue. Registering a value CAGR of 12.9% between 2017 and 2025, the Microbial segment will hold the second position among the cell type segments of the global GMP cell banking services market.
Planning To Introduce An Offbeat Product/Technology In The GMP Cell Banking Service Market? Go To "Purchase Now" To Have Our GMP Cell Banking Service Market Report! https://www.persistencemarketresearch.com/checkout/13735
Biopharmaceutical Companies will be the largest end users of GMP cell banking services
Among the end user segment of the global GMP cell banking services market, the Biopharmaceutical Companies segment will emerge the undisputed leader both in terms of market share (estimated to hold almost 69% share by 2017 end) and CAGR (an impressive 15.4% during 2017 - 2025). In comparison, the Contract Manufacturing Organizations segment will come a pale second, recording a market attractiveness index of 0.5 during the forecast period.
Access Related Reports-
Medical Spa Market: https://www.persistencemarketresearch.com/market-research/medical-spa-market.asp
Elder Care Services Market: https://www.persistencemarketresearch.com/market-research/elder-care-services-market.asp
Contact Us:
Persistence Market Research
Address - 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. - +1-646-568-7751
USA-Canada Toll-free - +1 800-961-0353
Sales - sales@persistencemarketresearch.com
Website - https://www.persistencemarketresearch.com
About Us:
Persistence Market Research (PMR), as a 3rd-party research organization, does operate through an exclusive amalgamation of market research and data analytics for helping businesses ride high, irrespective of the turbulence faced on the account of financial/natural crunches.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The GMP Cell Banking Service Market to Turn Explicit With Innovation here
News-ID: 2564883 • Views: …
More Releases from Persistence Market Research
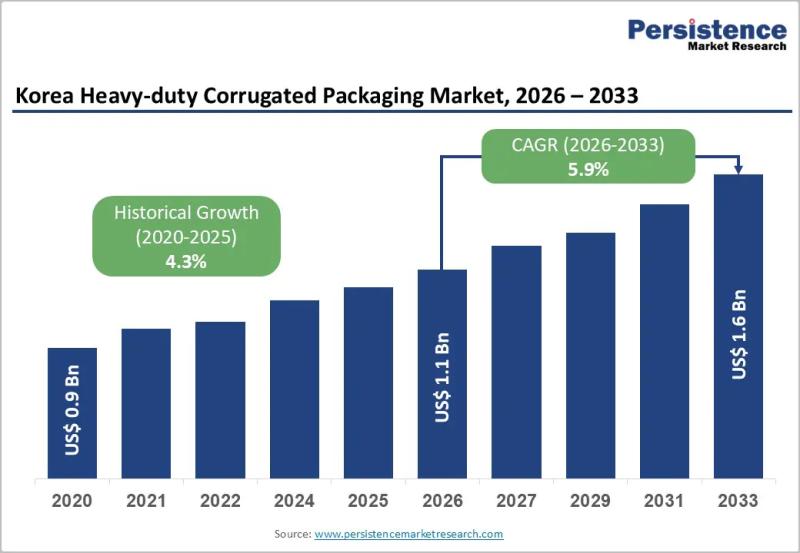
Korea Heavy-duty Corrugated Packaging Market to Reach US$1.6 Billion by 2033 - P …
The Korea heavy-duty corrugated packaging market plays a critical role in supporting industrial logistics, bulk transportation, and export-driven manufacturing. Heavy-duty corrugated packaging is widely used for shipping machinery, automotive components, electronics, chemicals, and large industrial goods that require superior strength and structural integrity. Unlike conventional corrugated boxes, heavy-duty variants are engineered with multi-wall boards, reinforced liners, and customized structural designs to withstand high load capacity, stacking pressure, and long-distance transportation.…

Textile Flooring Market Set for Steady Growth as Demand for Sustainable and Styl …
The global textile flooring market is entering a phase of stable expansion, supported by rising construction activity, increasing consumer focus on interior aesthetics, and growing demand for eco-friendly flooring solutions. According to industry estimates, the global textile flooring market size is likely to be valued at US$11.1 billion in 2026 and is projected to reach US$16.5 billion by 2033, expanding at a CAGR of 5.8% between 2026 and 2033. This…

Power System Simulator Market Size to Reach US$ 2.6 Billion by 2033 - Persistenc …
The power system simulator market is gaining strategic importance as global energy systems transition toward digitalization, decentralization, and decarbonization. Power system simulators are advanced software and hardware platforms used by utilities, grid operators, engineering firms, and research institutions to model, analyze, and optimize electrical power networks. These simulators enable real time grid analysis, contingency planning, load flow studies, fault analysis, stability assessment, and operator training. As electricity networks become more…

Yoga and Meditation Products Market Set for Robust Growth, Projected to Reach US …
The global wellness industry is undergoing a major transformation as consumers increasingly prioritize mental health, mindfulness, and preventive self-care. Within this evolving landscape, the yoga and meditation products market has emerged as a fast-growing segment, encompassing everything from yoga mats and apparel to meditation cushions, smart devices, and digital-enabled accessories. According to industry estimates, the global yoga meditation products market is projected to be valued at US$ 8.3 billion in…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…