Press release
The Growth Factors Market to Be Driven By the Use of AI in Diagnosis
The Growth Factors Market is slated to grow unstoppably in the years to come. The healthcare providers are, of late, making way for more specialized and timely treatment through same-day surgeries, outpatient surgeries, and likewise. ASCs (Ambulatory Surgical Centers) are preferred over conventional hospital settings. As such, cost-effective medical services could be provided. This type of customization is expected to take the healthcare vertical by storm in the future.As per Persistence Market Research's latest revised industry analysis, the global growth factors market was valued at over US$ 1.7 Bn in 2020, and is expected to exhibit a CAGR of close to 7% over the forecast period (2021-2031).
According to the World Health Organization (WHO), cancer caused around 9.9 million deaths worldwide in 2020. In addition, around 19.2 million people across the world were suffering from cancer. Also, increasing occurrence of autoimmune disorders is anticipated to drive demand for growth factors.
Rising prevalence of autoimmune diseases and cancer and the benefits of cytokine and growth factors therapy in the treatment of these diseases are expected to complement market growth over the coming years.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/4456
Growing research in the field of wound healing and skin regeneration is also expected to favor expansion of the global growth factors market. However, high cost associated with GMP grade growth factors is estimated to hamper market growth.
Acquisitions, collaborations, and partnership agreements are rife in this landscape:
PPD, Inc., a leading clinical research organization, was acquired by Thermo Fisher Scientific in April 2021, expanding the company's service offerings to pharma and biotech customers.
Merck and BioMed X Institute extended their collaboration to continue novel oncology and autoimmunity research in 2021.
In 2021, Lonza and ValenzaBio entered into a manufacturing agreement to rapidly advance VB421, an anti-IGF-1R antibody for autoimmune diseases.
Company Profiles:
Thermo Fisher Scientific
Lonza Group AG
Merck KGaA
General Electronics Company
F. Hoffmann-La Roche Ltd
Applied Biological Materials (abm), Inc.
Abcam plc.
Cell Signaling Technology, Inc.
Meridian Bioscience Inc.
Sartorius CellGenix GmbH
Bio-Techne.
Proteintech Group, Inc.
Miltenyi Biotec
Creative Bioarray
Akron Biotech
Sino Biological Inc.
Repligen corporation
LEADGENE BIOMEDICAL, INC.
PeproTech Inc.
Others
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/4456
Key Takeaways from Market Study
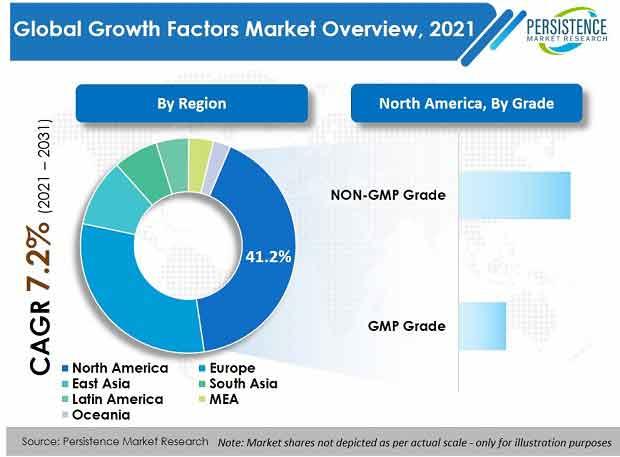
By grade, NON-GMP grade products hold around 70% market value share, globally, primarily due to increasing focus on oncology research and growing contract research activities.
Based on product, interleukins are leading with over 21% market share. Strong product pipelines by various key players are positively driving demand for interleukins among growth factor products.
Cell therapy and ex vivo manufacturing are estimated to dominate the market by application. This segment accounted for approximately 29% share of the market, primarily due to increase in acceptance of growth factors in cancer and autoimmune therapies
Pharmaceutical and biotechnology companies dominate with a market share of 48%. Growing number of R&D activities and increasing preference toward growth factors for gene therapy have had a positive impact on the market.
North America is set to dominate the global market with a value share of around 41%. Europe is slated to be the second-largest region with a value share of 30%.
"Rising prevalence of cancer, preference toward use of regenerative medicines, and new product launches & approvals are expected to drive market growth over the coming years," says an analyst of Persistence Market Research.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/4456
Demand-side Drivers:
Increasing focus on oncology research
Growing demand for growth factors and cytokines in stem cell biology
Increasing demand for growth factors in regenerative medicine
Market Opportunities:
Shift toward fast growing Asia Pacific market
Limited number of companies with sole focus on GMP grade products
Market Competition
New product approvals, launches, collaborations, agreements, and partnerships have emerged as the main growth strategy implemented by leading players. By focusing on these strategies, key market players are strengthening their existing product portfolios to expand their geographic footprints.
In 2020, the humankind human cell-expressed growth factor of Proteintech Group, Inc received an ISO 13485 Certificate.
In 2020, Akron Biotech signed an agreement to acquire Cytiva. This acquisition helps enhance its cGMP-compliant solutions and support the development of advanced therapy.
Complete Report Details@ https://www.persistencemarketresearch.com/market-research/growth-factors-market.asp
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the growth factors market in its latest study, presenting a historical demand assessment of 2016 - 2020 and projections for 2021 - 2031.
The research study is based on the product (transforming growth factors (TGF), epidermal growth factors (EGFs), platelet-derived growth factors (PDGFs), fibroblast growth factors (FGFs), insulin-like growth factors (IGFs), vascular endothelial growth factors (VEGFs), hepatocyte growth factors (HGFs), tumour necrosis factors (TNFs), interleukins, and others), grade (GMP grade and NON-GMP grade), application (oncology research, haematology research, wound healing research, dermatology research, cardiovascular disease & diabetes, cell therapy and ex vivo manufacturing, and others), and end user (pharmaceutical and biotechnology companies, research centres & academic institutes, and CMOs & CDMOs), across seven key regions of the world.
Related Reports:
Transcatheter Heart Valve Replacement Repair Market - https://www.persistencemarketresearch.com/market-research/transcatheter-heart-valve-replacement-repair-market.asp
Induced Pluripotent Stem Cells Market - https://www.persistencemarketresearch.com/market-research/induced-pluripotent-stem-cells-market.asp
Contact Us:
Persistence Market Research
Address - 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. - +1-646-568-7751
USA-Canada Toll-free - +1 800-961-0353
Sales - sales@persistencemarketresearch.com
Website - https://www.persistencemarketresearch.com
About Us:
Persistence Market Research is here to provide companies a one-stop solution with regards to bettering customer experience. It does engage in gathering appropriate feedback after getting through personalized customer interactions for adding value to customers' experience by acting as the "missing" link between "customer relationships" and "business outcomes'. The best possible returns are assured therein.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The Growth Factors Market to Be Driven By the Use of AI in Diagnosis here
News-ID: 2556938 • Views: …
More Releases from Persistence Market Research

Dog Collars, Leashes & Harnesses Market Set to Reach US$ 9.51 Bn by 2030
Introduction
The global dog collars, leashes & harnesses market has experienced notable growth in recent years, driven by the rising adoption of companion animals, increasing pet humanization, and growing awareness of pet safety and comfort. Dogs are increasingly regarded as family members, leading pet owners to invest in high-quality, durable, and aesthetically appealing accessories that enhance both functionality and style. Collars, leashes, and harnesses play a critical role in pet control,…

Music Tourism Market Analysis Highlights 5.5% CAGR Through 2032
The global music tourism market is set to witness consistent growth over the next decade, supported by the rising popularity of live music experiences and experiential travel. According to recent industry analysis, the global music tourism market size is likely to be valued at US$9.6 billion in 2025 and is projected to reach US$14.0 billion by 2032, expanding at a compound annual growth rate (CAGR) of 5.5% during the forecast…

Electronic Dance Music Market Analysis Highlights Robust Growth Through 2031
➤ Introduction
The global Electronic Dance Music (EDM) market has emerged as one of the most dynamic and influential segments within the broader music and entertainment industry. Characterized by high-energy beats, immersive live performances, and strong digital integration, EDM has evolved from an underground cultural movement into a mainstream global phenomenon. The genre's rapid adoption across music festivals, nightclubs, streaming platforms, and social media channels has significantly reshaped consumer listening habits…

Global Memory Market Set for Strong Growth Driven by AI and Data Centers
The global memory market is entering one of its most transformative growth cycles in decades. As digital ecosystems scale rapidly across artificial intelligence (AI), cloud computing, data centers, automotive electronics, and edge devices, memory technologies are becoming the backbone of modern computing infrastructure. From high-performance servers to connected vehicles and IoT endpoints, memory capacity, speed, and efficiency now directly influence system performance and competitiveness.
The global memory market size is likely…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…