Press release
STAQ Pharma and URSATEC cooperate on Desmopressin nasal spray replacement for STIMATE for hemophilia patients
Denver, USA / Tholey, Germany – December 15th, 2021Recently, the only Desmopressin Acetate in an intranasal spray drug product was voluntarily recalled in the United States with an estimate to return to market of 2023. Hemophilia patients, and especially “von Willebrand’s” patients, were left with invasive treatment options that are less than ideal and costly.
The Food and Drug Administration quickly moved to have the intranasal spray placed on the Drug Shortage list and STAQ Pharma partnered with the Hemophilia Alliance to produce the product.
“Fortunately, as an FDA registered 503B outsourcing facility, STAQ Pharma was able to step into this critical drug shortage and provide hemophilia patients with a cGMP manufactured desmopressin nasal spray product,” said Joe Bagan, STAQ Pharma CEO.
STAQ Pharma worked with outside labs to ensure a proper drug formulation and stability study that would meet CGMP standards. “We conducted a multi-month process to evaluate world leaders in nasal spray devices, and URSATEC was at the top of our list. When we contacted URSATEC, they were supportive of the situation facing patients and quickly partnered with STAQ to provide an industry leading nasal spray device to help bring the product to market” said STAQ Pharma Chief Pharmacist Jeff Hval.
A key advantage in the cooperation between URSATEC and STAQ is that with URSATECs established and proven technology, the nasal spray was developed in the quickest time possible. With state-of-the-art laboratories and highest-grade production lines, URSATEC was able to supply a matching primary packaging for the top-quality product STIMATETM.
For URSATEC this project is an important step to further increase their field of activity and market their technologies for nasal sprays in the USA.
“We are very proud of the cooperation together with STAQ, and to take another step to become a renowned partner for American innovators in the pharmaceutical market. These kind of products help to spread awareness of the technological possibilities and may cause a change of view when it comes to new product developments.” Said URSATECs CEO Dr. Andreas Bilstein.
Interested companies can find more details on the respective websites
www.staqpharma.com as well on www.ursatec.com/en
Dr. Andreas Bilstein
URSATEC GmbH
Marpinger Weg 4
66636 Tholey
Germany
Phone: phone: +49 (0) 6853-96199 0
fax: +49 (0) 6853-96199 14
bilstein@ursatec.com
www.ursatec.com
About STAQ
Founded in 2018 in Denver, Colorado, STAQ is proud to be one of the country’s first 503Bs designed and built from the ground up to comply specifically with FDA regulations as a cGMP facility.
With a cGMP-focused 503B facility, doctors and their patients have an added level of safety and confidence when using STAQ products. As the name is short for “Safety, Transparency, Availability, Quality “, STAQ provides top quality pharmaceutical compounding with state-of-the-art manufacturing.
About Ursatec
Ursatec GmbH was founded in 1993 as a Spin-off from Aero Pump, Gaplast and Ursapharm to accomplish one mission: the establishment of preservative-free applications, based on its proprietary packaging systems in different application areas. Having sold almost two billion units within the last 25 years, the company became the market leader in this segment and is proud to have founded a rethinking in the market for nasal sprays and set preservative-free formulations as the new standard in Europe. Ursatec is consistently expanding its business and develops and offers dosage systems, primary packaging materials, filling services, white-label OTC medical devices, food supplements and cosmetics on a B2B basis to the healthcare industry.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release STAQ Pharma and URSATEC cooperate on Desmopressin nasal spray replacement for STIMATE for hemophilia patients here
News-ID: 2499252 • Views: …
More Releases from Ursatec GmbH
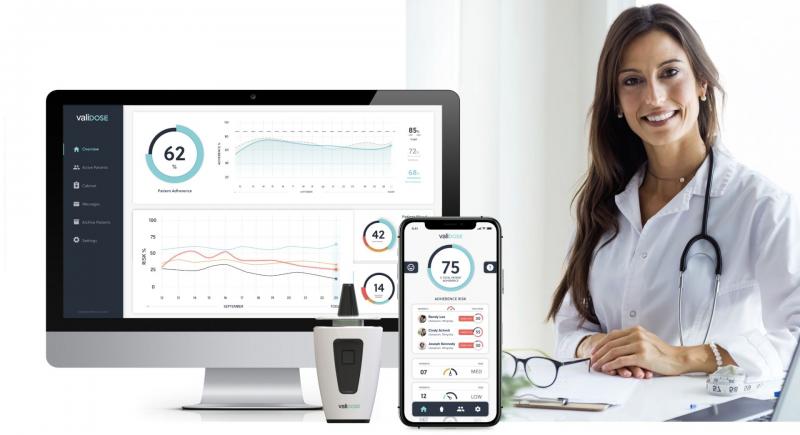
Aero Pump, Validose and Ursatec cooperate on digital augmentation of pump-based …
Hochheim/New York/Tholey, Nov. 2, 2021. Aero Pump GmbH, Validose, Inc. and Ursatec GmbH today announced a collaboration to explore the usage of Validose´s biometrically secure intranasal medical device that optimizes drug delivery, prevents medication misuse and allows telemedicine and other digital facilitated control of the application, together with the market leading nasal spray systems from Aero Pump and Ursatec, targeting preserved and preservative-free nasal spray solutions.
Marcel Botha, CEO of…
More Releases for STAQ
Global Bakery Processing Equipment Market to Reach US$ 22.5 Billion by 2035 Repo …
The bakery industry is undergoing a major technological and structural transformation. As global consumers increasingly crave convenient, high-quality, and specialty baked products, the Bakery Processing Equipment Market is witnessing significant expansion. Valued at US$ 14.3 billion in 2024, the market is projected to grow at a compound annual growth rate (CAGR) of 7.9% from 2025 to 2035, ultimately reaching US$ 22.5 billion by the end of the forecast period.
The rise…