Press release
Porphyria Targeting Therapies Market, 2021-2030
Roots Analysis has announced the addition of “Porphyria Targeting Therapies Market, 2021-2030” report to its list of offerings.Porphyria is a rare disorder that is characterized by excessive accumulation of porphyrin, a compound that aids in the formation of heme (an essential part of hemoglobin that helps carry oxygen in blood). Any anomaly caused by genetic or acquired abnormalities in heme biosynthesis (produced majorly in bone marrow and liver) can result in toxicity. It is worth highlighting that, till date, more than 1,000 mutations that can cause porphyria have been identified. However, prevalence of porphyria still remains unknown. Several treatment options such as gene therapy, proteasome inhibition and pharmacologic chaperones are currently being investigated among various other targeted treatment options.
To order this 130+ slides report, which features 90+ figures, please visit https://www.rootsanalysis.com/reports/porphyria-pipline-review.html.
Key Market Insights
15+ therapies have been / are being developed for the treatment of different types of porphyria
More than 70% of the aforementioned candidates are currently under clinical evaluation. Further, three therapies, namely Panhematin™, GIVLAARI® and SCENESSE®, have already been approved for the treatment of different types of porphyria.
Around 50% of the therapeutics are being developed as biologics
Majority (over 65%) of the abovementioned biologic drugs have been / are being designed for administration via the intravenous route. Furthermore, majority of the drugs (37%) have been / are being targeting acute intermittent porphyria.
Over 30% of the therapies have been / are being developed for erythropoietic protoporphyria
More than 65% the abovementioned therapies are currently being evaluated in clinical phases. Further, around 60% of the aforementioned therapy candidates are being developed as small molecules.
More than 45% of the players evaluating therapies for porphyria are small companies
North America has emerged as a key hub for the development of porphyria therapies, featuring the presence of 65% developers. The developer landscape is further dominated by players that have been established between 2001-2010, representing around 45% of the total number of stakeholders.
A number of clinical trials evaluating therapies for porphyria, have been registered
Majority of the clinical studies have been completed. More than 30% of the overall trials are phase I studies. Further, it is worth noting that, most of the trials (~ 60%) focused on porphyria therapies were registered post-2010.
To order this 130+ slides report, which features 90+ figures, please visit https://www.rootsanalysis.com/reports/porphyria-pipline-review.html.
Partnership activity in this field has increased at a CAGR of 9.6%, between 2018 and 2020
More than 70% of the reported deals were established post-2018, with the maximum activity being reported in 2019 and 2020. Majority of the instances captured in the report were product distribution / commercialization agreements (~45%).
380+ articles have been published related to porphyria, since January 2018
Close to 20% publications mentioned in the report were focused on the assessment of therapeutics that have been / are being developed for the treatment of erythropoietic protoporphyria. Example of prominent journals include (in decreasing order of number of publications) Molecular Genetics, Orphanet Journal of Rare Diseases, British Journal of Dermatology and Molecular Genetics, and Metabolism Reports.
Around 15 eminent individuals were identified as key opinion leaders (KOLs) in this domain
More than 65% of these KOLs were observed to be associated with organizations based in US, followed by those affiliated to institutes in Spain (20%) and South Africa (7%). Further, over 65% of the KOLs are currently affiliated to academic institutes, such as schools and universities.
North America is anticipated to capture over 60% of the global market share in 2030
In 2030, more than 50% of the market revenues are expected to be generated from sales of therapeutics intended for the treatment of erythropoietic protoporphyria and porphyria cutanea tarda. Further, therapies designed for oral route of administration are expected to occupy a larger share (51%) of the overall market, in the foreseen future.
To request a sample copy / brochure of this report, please visit this link.
Key Questions Answered
What are the prevalent R&D trends related to Porphyria?
What are the key challenges faced by stakeholders engaged in this domain?
What are the principal therapies developed by the companies in this domain?
Who are the leading industry and non-industry players in this market?
What are the key geographies where research on porphyria is actively being conducted?
Who are the key investors in this domain?
Who are the key opinion leaders / experts in this field?
What kind of partnership models are commonly adopted by industry stakeholders?
What are the factors that are likely to influence the evolution of this upcoming market?
How is the current and future market opportunity likely to be distributed across key market segments?
The financial opportunity within the porphyria therapies market has been analyzed across the following segments:
Drug
GIVLAARI®
Panhematin®
SCENESSE®
MT-7117
Colestid
HARVONI®
Type of Porphyria
Acute Hepatic Porphyria
Acute Intermittent Porphyria
Erythropoietic Protoporphyria
Hereditary Coproporphyria
Porphyria Cutanea Tarda
Variegate Porphyria
X-Linked Porphyria
Route of Administration
Oral
Intravenous
Subcutaneous
Key Geographical Regions
North America
Europe
Asia-Pacific
The research includes profiles of key players (listed below); each profile features a brief overview of company, pipeline details, recent developments (including collaborations and expansions) and an informed future outlook.
Agios Pharmaceutical
Alnylam Pharmaceuticals
Clinuvel Pharmaceuticals
Disc Medicine
Mitsubishi Tanabe Pharma
Moderna Therapeutics
Palatin Technologies
Recordati Rare Diseases
For additional details, please visit https://www.rootsanalysis.com/reports/porphyria-pipline-review.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Progressive Supranuclear Palsy: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2030
2. Soft Tissue Sarcoma: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2030
3. Polycystic Ovarian Syndrome (PCOS): Pipeline Review, Developer Landscape and Competitive Insights, 2021-2030
Contact Details
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com
Roots Analysis
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis.com
Medium: https://medium.com/@RootsAnalysis
Pinterest: https://in.pinterest.com/RootsanalysisPin/_saved/
Quora: https://rootsanalysisinsights.quora.com/
Contact Details
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com
Roots Analysis
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis.com
Medium: https://medium.com/@RootsAnalysis
Pinterest: https://in.pinterest.com/RootsanalysisPin/_saved/
Quora: https://rootsanalysisinsights.quora.com/
We specialise in analysing areas which have lacked quality research so far or require more focussed understanding within the broader industry. All our reports are structured in a way to enable the reader develop a thorough perspective on the given subject. Apart from writing reports on identified areas, we also provide bespoke research / consulting services dedicated to serve our clients in the best possible way.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Porphyria Targeting Therapies Market, 2021-2030 here
News-ID: 2478736 • Views: …
More Releases from Roots Analysis

Biologics Contract Manufacturing Market Size Worth over USD 55 Billion in 2035 | …
Global Biologics Contract Manufacturing Market Overview
The biologics contract manufacturing market, valued at USD 21.2 billion in 2024, is projected to grow to USD 23.8 billion in 2025 and USD 55.0 billion by 2035, representing a CAGR of 8.8% during the forecast period.
Various challenges associated with the development process of biologics, such as complex stages of manufacturing, limitations of analytical sciences, lack of incentives and the current statutory and regulatory framework,…
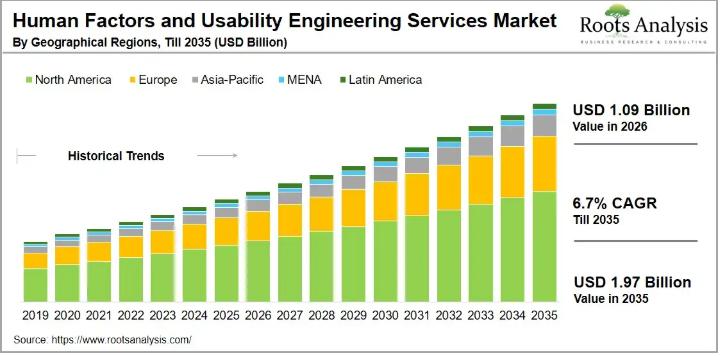
Human Factors and Usability Engineering Services Market CAGR To be ~7% During Th …
The global human factors and usability engineering services market, valued at USD 1.01 billion in 2025, is projected to reach USD 1.09 billion in 2026 and USD 1.97 billion by 2035, with a 6.7% CAGR during the forecast period 2026 to 2035.
In recent years, there has been a noticeable rise in user awareness towards healthcare-related harm arising due to the error caused by the medical devices. The key strategy for…
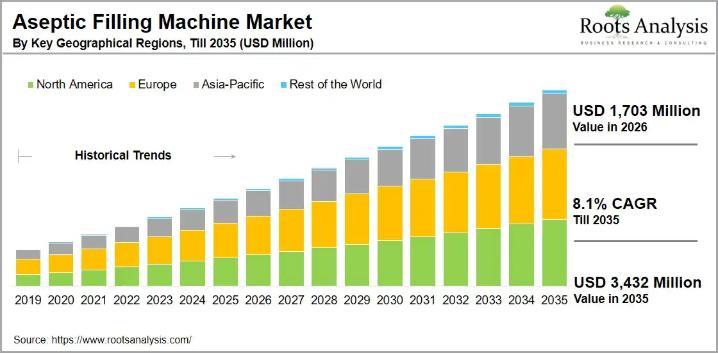
Isolator based Aseptic Filling Machine Market Size Worth over USD 3.4 Billion in …
Global Isolator based Aseptic Filling Machine Market Overview
The global aseptic filling machine market size, valued at USD 1,531 million in 2025, is projected to reach USD 1,703 million in 2026 and USD 3,432 million by 2035, representing a CAGR of 8.1% during the forecast period 2026 to 2035.
Several factors contribute to the growth of the isolator based aseptic filling machine market. Fill / finish is considered as a critical aspect…

Proteomics Market Size Worth over USD 94.7 Billion in 2035 | Roots Analysis
Global Proteomics Market Overview
The global proteomics market, valued at USD 31.6 billion in 2024, is project to reach USD 35.7 billion in 2025 and USD 94.7 billion by 2035, representing a CAGR of 10.2% during the forecast period 2025 to 2035.
Proteomics is a new type of 'omics', aiding in understanding the structure and function of different proteins as well as protein-protein interactions. It is worth noting that a variety of…
More Releases for Porphyria
Porphyria Targeting Therapies Market Trends, Pipeline Insights, and Forecast
The Porphyria Targeting Therapies Market is a niche segment within the broader field of rare diseases and specialized therapeutic interventions. Porphyria refers to a group of rare, inherited disorders that result from a buildup of porphyrins in the body, leading to various health complications, including abdominal pain, neurological issues, and skin sensitivity. The targeting therapies for Porphyria aim to address these conditions by either correcting metabolic defects or alleviating the…
What's Driving the Acute Intermittent Porphyria Market 2025-2034: Impact Of Risi …
What Are the Projections for the Size and Growth Rate of the Acute Intermittent Porphyria Market?
There has been a robust growth in the market size of acute intermittent porphyria in the past few years. The market that was valued at $4.37 billion in 2024, is projected to increase to $4.66 billion in 2025, marking a compound annual growth rate (CAGR) of 6.7%. Factors such as increasing healthcare spending, a surge…
Acute Intermittent Porphyria Market Acute Intermittent Porphyria Treatments, Siz …
According to a new report published by CoherentMI The acute intermittent porphyria market is estimated to be valued at USD 1.43 Billion in 2024 and is expected to reach USD 2.17 Billion by 2031, growing at a compound annual growth rate (CAGR) of 6.1% from 2024 to 2031.
The Global Acute Intermittent Porphyria Market has recently been analyzed and explored by CoherentMI in their latest market research report. The…
Enhance Genetic Testing Module for Acute Intermittent Porphyria
The "Acute Intermittent Porphyria Market" is a dynamic and rapidly evolving sector, with significant advancements and growth anticipated by 2031. Comprehensive market research reveals a detailed analysis of market size, share, and trends, providing valuable insights into its expansion. This report delves into segmentation and definition, offering a clear understanding of market components and drivers. Employing SWOT and PESTEL analyses, the study evaluates the market's strengths, weaknesses, opportunities, and threats,…
Porphyria Therapeutics- Pipeline Analysis 2018, Clinical Trials & Results | Alny …
Porphyria is a group of disorders including cutaneous porphyria and acute porphyria. Cutaneous porphyria affects the skin while acute porphyria affects the nervous system. Porphyria cutanea tarda is the most common type of porphyria prevalent in the U.S.
Download the sample report at: https://www.pharmaproff.com/request-sample/1043
The common symptoms of porphyria are abdominal pain, chest pain, increased blood pressure, increased heart rate, muscle weakness, cramping, blisters, itching, swelling, constipation, vomiting, mental disorders,…
Global Swedish Porphyria Industry Assesment report 2018-2025
Swedish porphyria is a rare autosomal dominant metabolic disorder affecting the production of heme resulting from a deficiency of the porphobilinogen deaminase. It is the most common of the acute porphyrias.
In 2018, the global Swedish Porphyria market size was xx million US$ and is forecast to xx million US in 2025, growing at a CAGR of xx% from 2018. In this study, 2017 has been considered as the base year…
