Press release
In Vitro Diagnostics (IVD) Quality Control Market worth $1.4 billion by 2026 - Exclusive Report by MarketsandMarkets™
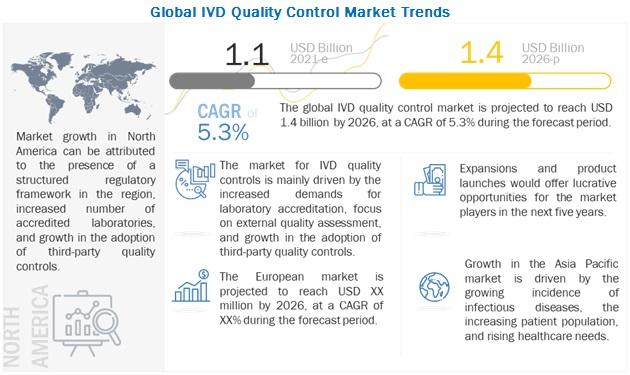
According to the new market research report "In Vitro Diagnostics (IVD) Quality Control Market by Source (Plasma, Whole Blood, Urine), Technology (Immunoassay, Hematology, Microbiology, Molecular Diagnostics), Manufacturer (Third-party, OEM), End Users (Hospitals, Lab) - Global Forecast to 2026", published by MarketsandMarkets™, the global IVD Quality Control Market is projected to reach USD 1.4 billion by 2026 from USD 1.1 billion in 2021, at a CAGR of 5.3% during the forecast period.Browse in-depth TOC on "In Vitro Diagnostics (IVD) Quality Control Market"
252 – Tables
59 – Figures
342 – Pages
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=198032582
The IVD testing plays a significant role in clinical decision-making. Over the years, IVD quality control products and procedures have become mandatory in accredited clinical or medical laboratories. The applications of IVD quality control products have also widened over the years, with a number of quality controls currently available in the market for clinical chemistry, immunochemistry, hematology, molecular diagnostics, coagulation, and microbiology. To ensure technological competitiveness, companies are continuously launching innovative and advanced quality control products in the market for a variety of applications.
The growth of the In Vitro Diagnostics Quality Control Market is primarily driven by the rising number of accredited clinical laboratories, rising geriatric population, rising demand for external quality assessment programs, increasing adoption of and POC instruments in developed regions and increasing adoption of third-party quality controls. The rising focus on multi-analyte controls is also expected to offer significant growth opportunities for the market in the coming years. The use of quality control products is, however, not mandatory for all clinical laboratories in many countries. The lack of regulations for these products is expected to adversely affect market growth.
The product & service segment holds the highest share of the total IVD quality control market during the forecast period.
Based on product & service, the In Vitro Diagnostics Quality Control Market is segmented into quality control products, data management solutions, and quality assurance services. The quality control products segment accounted for the largest share of the market in 2020. The increasing number of accredited laboratories and mandates for the use of quality controls from regulatory bodies to ensure the accuracy of diagnostic test results are driving the growth of the IVD quality control products market.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=198032582
Immunochemistry accounted for the highest share of the technology segment of the global IVD quality control market
Based on technology, the In Vitro Diagnostics Quality Control Market is broadly segmented into clinical chemistry, immunochemistry, hematology, coagulation & hemostasis, microbiology, molecular diagnostics, and other technologies. The immunochemistry segment accounted for the largest share of the market in 2020. The large share of this segment can be attributed to the increasing use of multi-analyte controls to perform immunoassay tests in laboratories. The use of immunoassay tests in clinical laboratories has also increased due to the high sensitivity of these tests over conventional methods.
Third-party controls accounted for the largest share for the IVD quality control market
Based on manufacturer, the In Vitro Diagnostics Quality Control Market is segmented into third-party controls and OEM controls. The third-party controls segment accounted for the largest share of the global market in 2020. The large share of this segment can be attributed to the increasing use of third-party quality controls across the globe to verify the accuracy and reliability of tests
Hospitals accounted for the highest share of the global IVD quality control market
The key end users of IVD quality controls studied in this report include hospitals, clinical laboratories, research & academic institutes, and other end users. The hospitals segment accounted for the largest share of the market in 2020, owing to the large volume of diagnostic tests carried out in hospitals.
Speak to Analyst: https://www.marketsandmarkets.com/speaktoanalystNew.asp?id=198032582
North America is expected to account for the largest share for players operating in the global IVD quality control market
Geographically, the global In Vitro Diagnostics Quality Control Market studied in this report is divided into five major regions— North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2020, North America accounted for the largest share of the global market, followed by Europe. Recommendations for and approvals of quality control products from the FDA and the College of American Pathologists (CAP) and the presence of well-established distribution channels and leading companies in the US are driving the market in North America.
Some of the key players in the In Vitro Diagnostics (IVD) Quality Control Market include Bio-Rad Laboratories, Inc. (US), Randox Laboratories Ltd. (UK), Thermo Fisher Scientific, Inc. (US), LGC Limited (UK), and Abbott Laboratories (US). Other prominent payers in the market include Roche Diagnostics (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), Fortress Diagnostics (UK), SERO AS (US), Sysmex Corporation (Japan), Ortho-Clinical Diagnostics (US), Helena Laboratories Corporation (US), Quidel Corporation (US), Sun Diagnostics, LLC (US), Seegene Inc. (South Korea), ZeptoMetrix Corporation (US), Qnostics (UK), Bio-Techne Corporation (US), Microbiologics (US), Microbix Biosystems (Canada), Streck, Inc. (US), Alpha-Tec Systems (US), Maine Molecular Quality Controls, Inc. (US), and Grifols, S.A. (Spain). These players aim to secure higher market shares through strategies such as product launches, expansions, agreements, and acquisitions.
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA: +1-888-600-6441
MarketsandMarkets™ provides quantified B2B research on 30,000 high growth niche opportunities/threats which will impact 70% to 80% of worldwide companies' revenues. Currently servicing 7500 customers worldwide including 80% of global Fortune 1000 companies as clients. Almost 75,000 top officers across eight industries worldwide approach MarketsandMarkets™ for their painpoints around revenues decisions.
Our 850 fulltime analyst and SMEs at MarketsandMarkets™ are tracking global high growth markets following the "Growth Engagement Model – GEM". The GEM aims at proactive collaboration with the clients to identify new opportunities, identify most important customers, write "Attack, avoid and defend" strategies, identify sources of incremental revenues for both the company and its competitors. MarketsandMarkets™ now coming up with 1,500 MicroQuadrants (Positioning top players across leaders, emerging companies, innovators, strategic players) annually in high growth emerging segments. MarketsandMarkets™ is determined to benefit more than 10,000 companies this year for their revenue planning and help them take their innovations/disruptions early to the market by providing them research ahead of the curve.
MarketsandMarkets's flagship competitive intelligence and market research platform, "Knowledge Store" connects over 200,000 markets and entire value chains for deeper understanding of the unmet insights along with market sizing and forecasts of niche markets.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In Vitro Diagnostics (IVD) Quality Control Market worth $1.4 billion by 2026 - Exclusive Report by MarketsandMarkets™ here
News-ID: 2456875 • Views: …
More Releases from MarketsandMarkets

Top Ultrasound Market Trends Driving Growth in 2025 and Beyond | Philips Healthc …
The global ultrasound market is entering a transformative phase in 2025. Once primarily associated with pregnancy scans and basic imaging, ultrasound has now evolved into a powerful, multipurpose diagnostic tool with applications across cardiology, oncology, musculoskeletal care, emergency medicine, and beyond.
As healthcare systems worldwide shift towards non-invasive, affordable, and portable imaging solutions, ultrasound is becoming central to modern diagnostics. According to market insights, the ultrasound industry is poised for steady…
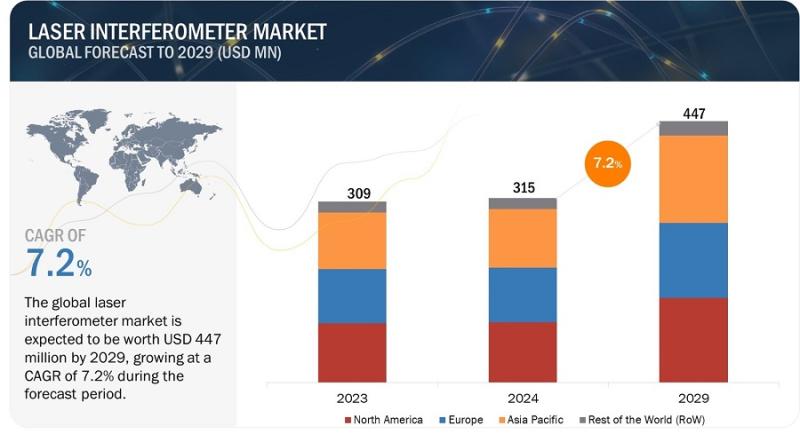
Laser Interferometer Market Set to Grow at the Fastest Rate- Time to Grow your R …
The global laser interferometer market is expected to be valued at 315 million in 2024 and is projected to reach USD 447 million by 2029, at a CAGR of 7.2% from 2024 to 2029. Emerging applications in industries push the market's growth due to the growing demand for precision in the manufacturing sector. However, challenges such as higher initial investments and maintenance costs cause problems. Despite these, opportunities arise for…
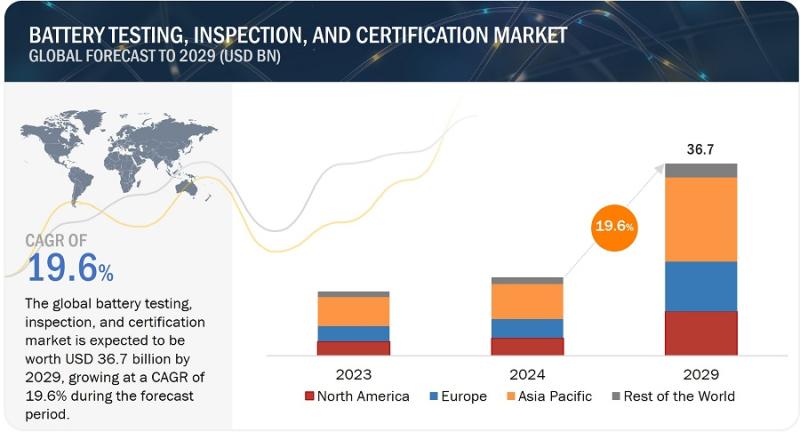
With 19.6% CAGR, Battery Testing, Inspection, and Certification Market Growth to …
The battery testing, inspection, and certification market is projected to reach USD 36.7 billion by 2029 from USD 14.9 billion in 2024 at a CAGR of 19.6% during the forecast period. Increasing adoption of EVs and energy storage systems, rising enforcement of stringent standards to ensure battery safety, thriving portable electronics industry, and rapid advances in battery technology are the major factors contributing to the market growth.
Download PDF Brochure @…
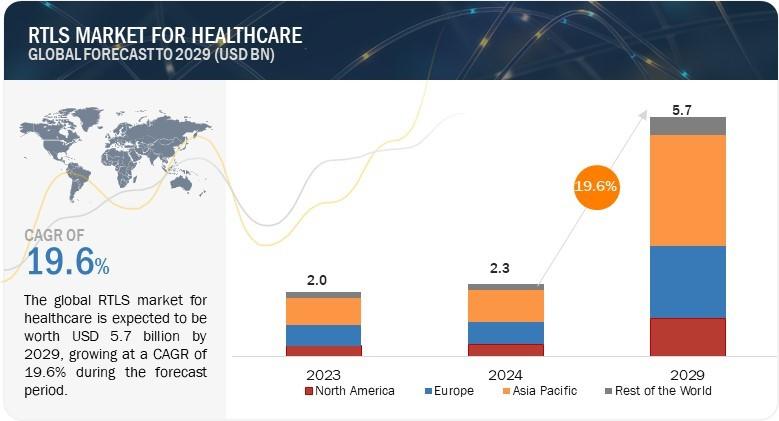
Real-Time Location Systems Revolutionize Healthcare: Insights from MarketsandMar …
The global RTLS market for healthcare is projected to grow from USD 2.3 billion in 2024 to USD 5.7 billion by 2029, at a compound annual growth rate of 19.6% from 2024 to 2029. As it attracts more and more players who enter this market with innovative RTLS features for customers, the market for RTLS technology is rapidly increasing. Top companies in this market focus on healthcare, retail, and manufacturing…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…