Press release
Peripheral Vascular Stents Market Report- Global Industry Analysis 2021-2026 | Nipro Corp, NSVascular, Inc
The Peripheral Vascular Stents Market research report enhances the dynamic cycle by understanding the methodologies that support business interest regarding customer product, market division, and forecast period. Peripheral Vascular Stents Marketalso examines the market’s political, economic, innovation, and social effects, particularly in North America, Europe, Asia Pacific, Middle East, Africa, and South America.
Get FREE PDF Sample of the Report @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4998935
Peripheral Vascular Stents Market Report provides comprehensive information about the Peripheral Vascular Stents pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
A peripheral vascular stent is an expandable perforated tube which is inserted into a peripheral vessel to prevent blood flow constriction. Bare metal stents (BMS), drug eluting stents (DES), covered stents and bio-absorbable stents are covered under this segment. A stent refers to a stent delivery system.
Scope of this Report
- Extensive coverage of the Peripheral Vascular Stents under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- The report reviews the major players involved in the development of Peripheral Vascular Stents and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy this Report-
Peripheral Vascular Stents Market Report enables you to -
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Peripheral Vascular Stents under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the products current stage of development, territory and estimated launch date
Get Discount on Purchase this Report @ https://www.reportsnreports.com/purchase.aspx?name=4998935
List of Tables
Peripheral Vascular Stents - Pipeline Products by Stage of Development
Peripheral Vascular Stents - Pipeline Products by Segment
Peripheral Vascular Stents - Pipeline Products by Territory
Peripheral Vascular Stents - Pipeline Products by Regulatory Path
Peripheral Vascular Stents - Pipeline Products by Estimated Approval Date
Peripheral Vascular Stents - Ongoing Clinical Trials
Peripheral Vascular Stents Companies - Pipeline Products by Stage of Development
Peripheral Vascular Stents - Pipeline Products by Stage of Development
3D Biotek LLC Pipeline Products & Ongoing Clinical Trials Overview
Bioresorbable Drug Eluting Peripheral Polymer Stent - Product Status
Bioresorbable Drug Eluting Peripheral Polymer Stent - Product Description
Abbott Vascular Inc Pipeline Products & Ongoing Clinical Trials Overview
ESPRIT Bioresorbable Vascular Scaffold - Product Status
ESPRIT Bioresorbable Vascular Scaffold - Product Description
ESPRIT BTK Bioresorbable Vascular Scaffold - Product Status
ESPRIT BTK Bioresorbable Vascular Scaffold - Product Description
Abbott Vascular Inc - Ongoing Clinical Trials Overview
ESPRIT BTK Bioresorbable Vascular Scaffold - LIFE-BTK (Pivotal Investigation of Safety and Efficacy of BRS Treatment-below the Knee) Randomized Controlled Trial
AlviMedica Medical Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
Cre8 BTK - Product Status
Cre8 BTK - Product Description
Easy Flype - Product Status
Easy Flype - Product Description
Easy HiFlype - Product Status
Easy HiFlype - Product Description
Isthmus Logic - Product Status
Isthmus Logic - Product Description
NiTiDES - Product Status
NiTiDES - Product Description
amg International GmbH Pipeline Products & Ongoing Clinical Trials Overview
UNITY-B Peripheral Stent - Product Status
UNITY-B Peripheral Stent - Product Description
AndraTec GmbH Pipeline Products & Ongoing Clinical Trials Overview
Pillar Bifurcation Reconstruction Device - Product Status
Pillar Bifurcation Reconstruction Device - Product Description
Arterius Ltd Pipeline Products & Ongoing Clinical Trials Overview
Arteriosorb Absorbable Drug-Eluting Scaffold - Product Status
Arteriosorb Absorbable Drug-Eluting Scaffold - Product Description
Bioresorbable Stent - Peripheral Vascular Disease - Product Status
Bioresorbable Stent - Peripheral Vascular Disease - Product Description
Biotyx Medical Pipeline Products & Ongoing Clinical Trials Overview
IBS Titan - Product Status
IBS Titan - Product Description
Biotyx Medical - Ongoing Clinical Trials Overview
IBS Titan - A Prospective, Multi-Center, Randomized Trial Comparing the IBS Titan Sirolimus-Eluting Iron Bioresorbable Peripheral Scaffold System vs. Standard Balloon Angioplasty for Treatment of Below-the-Knee Arteries: GENIUS TRIAL
Boston Scientific Corp Pipeline Products & Ongoing Clinical Trials Overview
Eluvia Drug-Eluting Vascular Stent System - Product Status
Eluvia Drug-Eluting Vascular Stent System - Product Description
SAVAL Drug Eluting Stent BTK - Product Status
SAVAL Drug Eluting Stent BTK - Product Description
Symbiot Covered Stent System - Product Status
Symbiot Covered Stent System - Product Description
Wallstent RP Endoprosthesis - Venous Outflow Obstruction - Product Status
Wallstent RP Endoprosthesis - Venous Outflow Obstruction - Product Description
Boston Scientific Corp - Ongoing Clinical Trials Overview
Eluvia Drug-Eluting Vascular Stent System - A Randomized Trial Comparing the ELUVIA Drug-eluting Stent Versus Bare Metal Self-expanding Nitinol Stents in the Treatment of Superficial Femoral and/or Proximal Popliteal Arteries
Eluvia Drug-Eluting Vascular Stent System - A Randomized Trial Comparing the ELUVIA Drug-eluting Stent Versus Zilver PTX Stent for Treatment of Superficial Femoral and/or Proximal Popliteal Arteries
Eluvia Drug-Eluting Vascular Stent System - A Real World Evaluation of the ELUVIA Drug Eluting Stent in All-comers with Superficial Femoral Artery and Proximal Popliteal Artery Disease
Eluvia Drug-Eluting Vascular Stent System - Drug-eluting Registry: Real-world Treatment of Lesions in the Peripheral Vasculature (ELEGANCE)
Eluvia Drug-Eluting Vascular Stent System - Sequent Please Drug Coated Balloons Versus Primary Stent Application in Long SFA Lesions
Wallstent RP Endoprosthesis - Venous Outflow Obstruction - Sinai Vein Stent Registry
SAVAL Drug Eluting Stent BTK - A Randomized Trial Comparing the Drug-eluting Stent (DES) Below-the-knee (BTK) Vascular Stent System (DES BTK Vascular Stent System) Versus Percutaneous Transluminal Angioplasty (PTA) Treating Infrapopliteal Lesions in Subjects with Critical Limb Ischemia
Cardiatis SA Pipeline Products & Ongoing Clinical Trials Overview
Cardiatis FluidSmart 3D Stent - Product Status
Cardiatis FluidSmart 3D Stent - Product Description
Cardinal Health Inc Pipeline Products & Ongoing Clinical Trials Overview
Palmaz-Mullins XD - Product Status
Palmaz-Mullins XD - Product Description
Cook Medical Inc Pipeline Products & Ongoing Clinical Trials Overview
Zenith Connection Endovascular Covered Stent - Product Status
Zenith Connection Endovascular Covered Stent - Product Description
Cordis Corp Pipeline Products & Ongoing Clinical Trials Overview
FlexStent Self Expanding Below-The-Knee Venous Stent System - Product Status
FlexStent Self Expanding Below-The-Knee Venous Stent System - Product Description
FlexStent Self Expanding Venous Stent System - Product Status
FlexStent Self Expanding Venous Stent System - Product Description
Tibial SE FlexStent - Product Status
Tibial SE FlexStent - Product Description
Corinnova Inc Pipeline Products & Ongoing Clinical Trials Overview
Hybrid Dynamic Stent - Product Status
Hybrid Dynamic Stent - Product Description
Cytograft Tissue Engineering Inc (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
LifeJacket Stent Graft - Peripheral - Product Status
LifeJacket Stent Graft - Peripheral - Product Description
Efemoral Medical LLC Pipeline Products & Ongoing Clinical Trials Overview
Efemoral Device - Product Status
Efemoral Device - Product Description
Efferent Labs Inc Pipeline Products & Ongoing Clinical Trials Overview
RTI-LICD Plexisense - Product Status
RTI-LICD Plexisense - Product Description
Electroformed Stents Inc Pipeline Products & Ongoing Clinical Trials Overview
Helical Stent - Product Status
Helical Stent - Product Description
Patch Stent - Product Status
Patch Stent - Product Description
Pleated Stent - Product Status
Pleated Stent - Product Description
Elixir Medical Corp Pipeline Products & Ongoing Clinical Trials Overview
PRAVA Bioresorbable Scaffold - Product Status
PRAVA Bioresorbable Scaffold - Product Description
FIT Biotech Oy (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
GTU X-Stent - Product Status
GTU X-Stent - Product Description
GTU X2-Stent - Product Status
GTU X2-Stent - Product Description
ID Nest Medical SAS Pipeline Products & Ongoing Clinical Trials Overview
ID Venous System - Product Status
ID Venous System - Product Description
InspireMD Inc Pipeline Products & Ongoing Clinical Trials Overview
PVGuard Peripheral - Product Status
PVGuard Peripheral - Product Description
Kyoto Medical Planning Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
REMEDY - Drug Eluting - Product Status
REMEDY - Drug Eluting - Product Description
REMEDY - New Version - Product Status
REMEDY - New Version - Product Description
LeoMed, LLC Pipeline Products & Ongoing Clinical Trials Overview
Peripheral Arterial Stent - Product Status
Peripheral Arterial Stent - Product Description
Lepu Medical Technology (Beijing) Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
Bare Stent - Product Status
Bare Stent - Product Description
LimFlow SA Pipeline Products & Ongoing Clinical Trials Overview
LimFlow Percutaneous Deep Vein Arterialisation System - Product Status
LimFlow Percutaneous Deep Vein Arterialisation System - Product Description
LimFlow SA - Ongoing Clinical Trials Overview
LimFlow Percutaneous Deep Vein Arterialisation System - Percutaneous Deep Vein Arterialization for the Treatment of Late-stage Chronic Limb-threatening Ischemia: The PROMISE II Trial
LimFlow Percutaneous Deep Vein Arterialisation System - Percutaneous Deep Vein Arterialization Post-market Study
LimFlow Percutaneous Deep Vein Arterialisation System - Percutaneous Deep Vein Arterialization Post-market Study
LimFlow Percutaneous Deep Vein Arterialisation System - Pilot Study to Investigate Safety, Effectiveness and Feasibility of LimFlow Stent Graft System for Creating an AV Fistula for the Treatment of Critical Limb Ischemia
Lyra Therapeutics Inc Pipeline Products & Ongoing Clinical Trials Overview
Stanza Bioresorbable Pediatric Scaffold - Product Status
Stanza Bioresorbable Pediatric Scaffold - Product Description
Stanza Bioresorbable Self-Expanding Scaffold - Product Status
Stanza Bioresorbable Self-Expanding Scaffold - Product Description
Medtronic Inc Pipeline Products & Ongoing Clinical Trials Overview
RADIANT - Product Status
RADIANT - Product Description
Meril Life Sciences Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview
Credence BRS - Product Status
Credence BRS - Product Description
Interlace BRS - Product Status
Interlace BRS - Product Description
Melange BRS - Product Status
Melange BRS - Product Description
Meril Life Sciences Pvt Ltd - Ongoing Clinical Trials Overview
Credence BRS - To evaluate safety and performance of CREDENCE BRS Sirolimus Eluting BioResorbable Peripheral Scaffold System in subjects with de novo native peripheral artery lesions
Melange BRS - A Prospective, Multicentre, Open Label Clinical Study to Evaluate Safety and Performance of Melange BRS Sirolimus Eluting Bioresorbable Peripheral Scaffold System for Use as an Adjunct to Percutaneous Transluminal Renal Angioplasty of Atherosclerotic De Novo or Restenotic Lesion of the Renal Artery
Micell Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
Peripheral Drug-Eluting Stent - Product Status
Peripheral Drug-Eluting Stent - Product Description
Micro Medical Solutions Inc Pipeline Products & Ongoing Clinical Trials Overview
MicroStent - Product Status
MicroStent - Product Description
Micro Medical Solutions Inc - Ongoing Clinical Trials Overview
MicroStent - A Clinical Evaluation of the MicroStent Peripheral Vascular Stent in Subjects with Arterial Disease below the Knee: STAND
MicroStent - An All-comers Observational Study of the MicroStent Peripheral Vascular Stent System in Subjects with Peripheral Arterial Disease
MicroPort Endovascular (Shanghai) Co., Ltd. Pipeline Products & Ongoing Clinical Trials Overview
Venous Stent - Product Status
Venous Stent - Product Description
Natec Medical Ltd Pipeline Products & Ongoing Clinical Trials Overview
Marina - Product Status
Marina - Product Description
Nipro Corp Pipeline Products & Ongoing Clinical Trials Overview
Peripheral Stent - Product Status
Peripheral Stent - Product Description
NSVascular, Inc. Pipeline Products & Ongoing Clinical Trials Overview
Micropatterned TFN Stent - Product Status
Micropatterned TFN Stent - Product Description
OrbusNeich Pipeline Products & Ongoing Clinical Trials Overview
Small Vessel - BTA - Product Status
Small Vessel - BTA - Product Description
PeriTec Biosciences Ltd Pipeline Products & Ongoing Clinical Trials Overview
Periteneum-Lined Balloon-Expandable Stent - Product Status
Periteneum-Lined Balloon-Expandable Stent - Product Description
Peritoneum Lined Self-Expanding Stent - Product Status
Peritoneum Lined Self-Expanding Stent - Product Description
QualiMed Innovative Medizinprodukte GmbH Pipeline Products & Ongoing Clinical Trials Overview
SOZO With 0.014" PERSEUS-q PTA Balloon Catheter - Product Status
SOZO With 0.014" PERSEUS-q PTA Balloon Catheter - Product Description
Tracheobronchial Stent - Product Status
Tracheobronchial Stent - Product Description
Qvanteq AG Pipeline Products & Ongoing Clinical Trials Overview
Qstent Peripheral Stent - Product Status
Qstent Peripheral Stent - Product Description
Reflow Medical, Inc. Pipeline Products & Ongoing Clinical Trials Overview
Temporary Spur Stent System - Product Status
Temporary Spur Stent System - Product Description
Reflow Medical, Inc. - Ongoing Clinical Trials Overview
Temporary Spur Stent System - A Non-randomized Pilot Study of the Temporary Spur Stent System for the Treatment of Lesions Located in the Infrapopliteal Arteries Using a LIMUS-base Drug-coated Balloon (DEEPER LIMUS)
Temporary Spur Stent System - A Non-randomized Trial of the Temporary Spur Stent System for the Treatment of Lesions Located in the Infrapopliteal Arteries Outside of the United States
Shenzhen Salubris Pharmaceuticals Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
Iliac Vein Stent - Product Status
Iliac Vein Stent - Product Description
Lower Limb Arterial Drug-Loaded Stent - Product Status
Lower Limb Arterial Drug-Loaded Stent - Product Description
Suzhou Tianhong Shengjie Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
Iliac Vein Stent System - Product Status
Iliac Vein Stent System - Product Description
Synergy Flow Ltd Pipeline Products & Ongoing Clinical Trials Overview
ArtiStent - Product Status
ArtiStent - Product Description
Taewoong Medical Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
Niti-S Nagi Stent - Product Status
Niti-S Nagi Stent - Product Description
Taewoong Medical Co Ltd - Ongoing Clinical Trials Overview
Niti-S Nagi Stent - A Randomized Control Trial of Plastic Stents Vs. Nagi Bi-flanged Metal Stent for Endoscopic Ultrasound Guided Drainage of Walled-off Necrosis
Tepha Inc Pipeline Products & Ongoing Clinical Trials Overview
TephaFLEX Fully Absorbable Peripheral Stent - Product Status
TephaFLEX Fully Absorbable Peripheral Stent - Product Description
TissueGen Inc Pipeline Products & Ongoing Clinical Trials Overview
Archer Stent - Product Status
Archer Stent - Product Description
Tractivus SL Pipeline Products & Ongoing Clinical Trials Overview
Bacteriaphobic Tracheal Stent - Product Status
Bacteriaphobic Tracheal Stent - Product Description
University of Michigan Pipeline Products & Ongoing Clinical Trials Overview
Tracheal Stent - Product Status
Tracheal Stent - Product Description
University of Pittsburgh Pipeline Products & Ongoing Clinical Trials Overview
Dual Chamber Stent - Product Status
Dual Chamber Stent - Product Description
University of Pittsburgh Medical Center Pipeline Products & Ongoing Clinical Trials Overview
Retrievable Vascular Stent - Product Status
Retrievable Vascular Stent - Product Description
Vascular Bioresorbable Technologies Pipeline Products & Ongoing Clinical Trials Overview
Drug Eluting Scaffold - Peripheral Artery Disease - Product Status
Drug Eluting Scaffold - Peripheral Artery Disease - Product Description
Drug Free Pure Bioresorbable Scaffold - Peripheral Artery Disease - Product Status
Drug Free Pure Bioresorbable Scaffold - Peripheral Artery Disease - Product Description
Vascular Concepts Ltd Pipeline Products & Ongoing Clinical Trials Overview
Covered Peripheral Stent - Product Status
Covered Peripheral Stent - Product Description
Prograft SX - Product Status
Prograft SX - Product Description
Vascular Dynamics Ltd. Pipeline Products & Ongoing Clinical Trials Overview
MobiusHD Device - Product Status
MobiusHD Device - Product Description
Vascular Dynamics Ltd. - Ongoing Clinical Trials Overview
MobiusHD Device - A Feasibility Study Exploring the Effect of the MobiusHD in Patients with Heart Failure
MobiusHD Device - Controlling and Lowering Blood Pressure with the MobiusHD - A Prospective Multicenter Safety Study: CALM-FIM_US
MobiusHD Device - Controlling and Lowering Blood Pressure with the MobiusHD - Defining Efficacy Markers
MobiusHD Device - Controlling and Lowering Blood Pressure with the MobiusHD Device: Studying Effects in a Randomized Trial: CALM-START
MobiusHD Device - Controlling and Lowering Blood Pressure with the MobiusHD: CALM- 2
Vesper Medical Inc Pipeline Products & Ongoing Clinical Trials Overview
Vesper Duo Venous Stent System - Product Status
Vesper Duo Venous Stent System - Product Description
Vesper Medical Inc - Ongoing Clinical Trials Overview
Vesper Duo Venous Stent System - Venous Stent for the Iliofemoral Vein Investigational Clinical Trial Using the DUO Venous Stent System
Vinnova Pipeline Products & Ongoing Clinical Trials Overview
Grency Venous Stent System - Product Status
Grency Venous Stent System - Product Description
VueKlar Cardiovascular Ltd (Inactive) Pipeline Products & Ongoing Clinical Trials Overview
MR-Enhancing Balloon Expandable Stent - Product Status
MR-Enhancing Balloon Expandable Stent - Product Description
MR-Enhancing Self-Expanding Stent - Product Status
MR-Enhancing Self-Expanding Stent - Product Description
Wake Forest University Health Sciences Pipeline Products & Ongoing Clinical Trials Overview
Vascular Stent - Product Status
Vascular Stent - Product Description
Xenogenics Corporation Pipeline Products & Ongoing Clinical Trials Overview
Ideal BioStent - Peripheral - Product Status
Ideal BioStent - Peripheral - Product Description
Unison Peripheral Stent System - Product Status
Unison Peripheral Stent System - Product Description
Zorion Medical Pipeline Products & Ongoing Clinical Trials Overview
ZMED Absorbable Drug Eluting Stent - Product Status
ZMED Absorbable Drug Eluting Stent - Product Description
Zylox-Tonbridge Medical Technology Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
Multi-Segment Stent System - Product Status
Multi-Segment Stent System - Product Description
Zenflex Peripheral Stent System - Product Status
Zenflex Peripheral Stent System - Product Description
ZENFLEX Pro Peripheral Drug Eluting Stent System - Product Status
ZENFLEX Pro Peripheral Drug Eluting Stent System - Product Description
Zylox-Tonbridge Medical Technology Co Ltd - Ongoing Clinical Trials Overview
ZENFLEX Pro Peripheral Drug Eluting Stent System - Swedish Drug-elution Trial in Peripheral Arterial Disease - A Multicenter, Prospective Randomized Controlled Clinical Trial Based on the Swedish Vascular Registry (SWEDVASC) Platform
ZENFLEX Pro Peripheral Drug Eluting Stent System - The Safety and Efficacy of Drug Eluting Peripheral Vascular Stent System for the Treatment of Superficial Femoral Artery Stenosis and /or Occlusion: A Multi-center Stratified Randomized Single-blind Controlled Trial
Zenflex Peripheral Stent System - ZENFlex-registry to Evaluate the Outcome of Bare Metal Stent-assisted Angioplasty in the Treatment of Superficial Femoral and/or Proximal Popliteal Arteries
Glossary
+ 1 888 391 5441
sales@reportsandreports.com
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Peripheral Vascular Stents Market Report- Global Industry Analysis 2021-2026 | Nipro Corp, NSVascular, Inc here
News-ID: 2450758 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
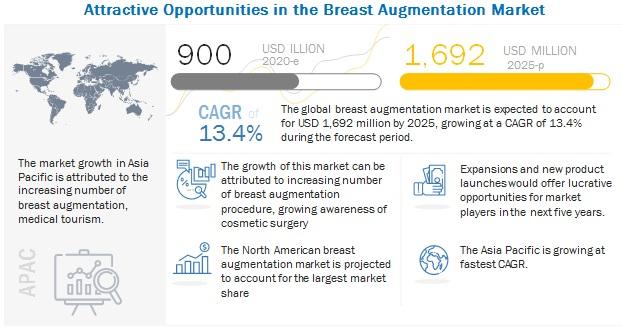
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Pro
WineCork Pro Reviews: Truth About WineCork Pro Revealed!!
There's nothing quite like the anticipation of opening a fine bottle of wine; whether you're celebrating a special moment, hosting friends, or winding down after a long day. But too often, that moment of joy is ruined by a stubborn cork, a broken opener, or the all-too-familiar embarrassment of struggling in front of guests. That's where WineCork Pro comes in; the smart, sleek solution that transforms wine opening from a…
BuzzZapper Pro Reviews: Is Buzz Zapper Pro Worth It?
BuzzZapper Pro Reviews: Is Buzz Zapper Pro Worth It?
If you've ever tried enjoying a quiet evening outdoors only to get eaten alive by mosquitoes, you'll understand exactly where I'm coming from. I've tried everything, from citronella candles to bug sprays that smell like a science experiment gone wrong. Some worked a little, most didn't, and all of them felt like a temporary fix.
That's when I came across the BuzzZapper Pro,…
Balmorex Pro: Does Balmorex Pro Really Works? Read The Balmorex Pro Reviews
Introduction
Joint and muscle aches can result from everyday wear and tear, aging, and poor nutrition. Whatever the reason, pain and ache issues are less than desirable. Who wants to suffer, rely on canes, or undergo surgery? No one, if they have a say in it.
With no guarantee of surgeries being successful and a high-risk rate, people have begun to rely on natural remedies that can prevent damage and start the…
ScrubClean Pro Reviews: Truth About ScrubClean Pro Scrubber Revealed
Based on ScrubClean Pro Reviews, it is one of the best spin power scrubber that is fairly priced. It is highly efficient with 4.9 stat ratings from the real users.
In this review, we are going to detail everything you might want to know about this innovative cleaning tools that is catching the market by storms. It is new but the amazing thing is that it is the number consumer choice.…
Pro Power Saver Reviews: is Pro Power Saver Legit? Read Pro Power Saver Consumer …
In recent years, the rising cost of energy bills has become a significant concern for both renters and homeowners in the United States. As the cost of living continues to increase, many individuals and families are searching for effective ways to reduce their monthly expenses. In response to this demand, a new device called Pro Power Saver has emerged as a trending solution in the country.
Pro Power Saver is an…
Pro Power Save (USA Update) Is Pro Power Save Legit? Pro Power save Reviews Cons …
Pro Power Save also known as Miracle Watt Energy Saver, the most trending energy saver in the United States with a stellar customer review rating of 4.8 out of 5.0. If you're tired of dealing with high electricity bills and want to save money, then this device might be the solution you've been searching for.
Pro Power Save is designed to help millions of users reduce their energy consumption when…