Press release
Novel Drug Reconstitution Systems Market, 2021-2030
Roots Analysis has announced the addition of “Novel Drug Reconstitution Systems Market, 2021-2030” report to its list of offerings.To order this 320+ page report, which features 230 figures and 248 tables, please visit https://www.rootsanalysis.com/reports/drug-reconstitution-systems-market.html
Key Inclusions
A detailed assessment of the current market landscape of novel drug reconstitution systems, providing information on the type of device (prefilled syringe, cartridge, infusion bags), type of chamber (dual chamber, multi chamber), physical state of drug (lyophilized, liquid), container fabrication material (glass, plastic), device usability (single use, multi-use), and volume of container. In addition, the chapter includes details related to novel drug reconstitution system manufacturers, along with information on their year of establishment, company size, location of headquarters and key players (in terms of number of products manufactured).
A detailed landscape of the reconstitution devices and systems featuring information on type of container or device, volume of primary container, physical state of drug, device usability and provision for self-administration. In addition, the chapter includes details related to the manufacturers, along with information on their year of establishment, company size and location of headquarters.
Elaborate profiles of prominent players engaged in this domain. Each profile includes a brief overview of the company, details related to its financial information (if available), information on product portfolio, recent developments and an informed future outlook.
A detailed analysis on the trends in packaging of over 350 drug products (including both biologics and small molecule drugs) that were approved by the FDA between 2014 and H1 2021, featuring an assessment of the packaging requirements of various container-closure systems based on several parameters, such as year of approval of drug, type of molecule (small molecule, biologic), type of biologic (allogeneic cell therapy, autologous cell therapy, fusion proteins, hormones, interferons, monoclonal antibodies, recombinant enzymes, recombinant protein and viral cell therapy), type of primary packaging container used (vials, pouches / packets, bottles, IV / sealed bags, prefilled syringes / pen , tubes, cartridge, blister packaging, others), type of packaging material(s) used for manufacturing primary container, type of closure used (cap / needle shield, seal, plunger, stopper and others), type of packaging material(s) used for manufacturing closures, dosage form, route of administration, holding temperature. In addition, the chapter provides information on the developers of the aforementioned drugs and an analysis based on year of establishment, company size, location of headquarters and leading drug developers (in terms of number of drugs approved).
An insightful analysis of the patents filed / granted for novel drug reconstitution systems, since 2011, taking into consideration various relevant parameters, such as type of patent, publication year, geographical location, CPC symbols, emerging focus areas, leading players (in terms of number of patents granted / filed in the given time period), patent characteristics and geography. In addition, the chapter includes a detailed patent benchmarking and an insightful valuation analysis.
A competitiveness analysis of novel drug reconstitution system manufacturers based on various relevant parameters, such as supplier power (in terms of experience / expertise of the manufacturer) and key product specifications (number of systems, type of systems, type of drugs and number of chambers).
An in-depth analysis of recent events (summits / forums / conferences / annual meetings) that were organized for stakeholders in this domain, highlighting the evolution of discussion topics related to novel drug reconstitution systems. The analysis also provides details on type of event, regional distribution, emerging agendas, popular organizers, active industry and non-industry players, and a schematic mapping of upcoming events.
A discussion on affiliated trends, key drivers and challenges, under a SWOT framework, featuring a Harvey ball analysis, highlighting the relative impact of each SWOT parameter on the overall novel drug reconstitution systems market.
An in-depth analysis to estimate the current and future demand for various novel drug reconstitution systems, including cartridges, infusion bags and prefilled syringes.
An elaborate discussion on emerging trends that are likely to have an impact on the future adoption of novel drug reconstitution systems. It presents a Harvey ball analysis, highlighting the relative effect of each trend on the adoption of novel drug reconstitution systems including dual chamber systems.
To order this 320+ page report, which features 230 figures and 248 tables, please visit https://www.rootsanalysis.com/reports/drug-reconstitution-systems-market.html
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
Type of Container
Prefilled Syringe
Cartridge
Infusion Bag
Fabrication Material
Glass
Plastic
Physical State of Drug in Syringe and Cartridge
Liquid / Powder
Liquid / Liquid
Physical State of Drug in Infusion Bag
Liquid Mixture
Frozen Mixture
Volume of Container
5 mL for prefilled syringe and cartridge
250 mL, 250-500 mL, 500-1,000 mL, >1,000 mL for infusion bag
Key Geographical Regions
North America
Europe
Asia-Pacific
Latin America
Middle East and North Africa
To request sample pages, please visit https://www.rootsanalysis.com/reports/drug-reconstitution-systems-market.html
Key Questions Answered
Who are the key players engaged in the development of novel drug reconstitution systems?
What is the relative competitiveness of different novel drug reconstitution system manufacturers?
What is the packaging trend in terms of container and closure for the drugs approved since 2014?
Who are the leading players focused on the development of lyophilized drugs?
What is the focus area of various conferences related to novel drug reconstitution systems?
How has the intellectual property landscape of novel drug reconstitution systems evolved over the years?
What are the emerging trends related to pharmaceutical packaging?
What are the key agenda items being discussed in various global events / conferences related to novel drug reconstitution systems?
How is the current and future market opportunity likely to be distributed across key market segments?
You may also be interested in the following titles:
1. Novel Ocular Drug Delivery Devices Market, 2021-2030
2. Pre-Sterilized / Ready-to-Use Primary Packaging Market, 2021-2030
3. Medical Device Batteries Market, 2020-2030
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
ben.johnson@rootsanalysis.com
Contact:
Gaurav Chaudhary
gaurav.chaudhary@rootsanalysis.com
Roots Analysis
A430, 4th Floor,
Bestech Business Towers, Sector 66, Mohali, India
sales@rootsanalysis.com
+1 (415) 800 3415
+44 (122) 391 1091
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis.com
We specialise in analysing areas which have lacked quality research so far or require more focussed understanding within the broader industry. All our reports are structured in a way to enable the reader develop a thorough perspective on the given subject. Apart from writing reports on identified areas, we also provide bespoke research / consulting services dedicated to serve our clients in the best possible way.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Novel Drug Reconstitution Systems Market, 2021-2030 here
News-ID: 2430526 • Views: …
More Releases from Roots Analysis
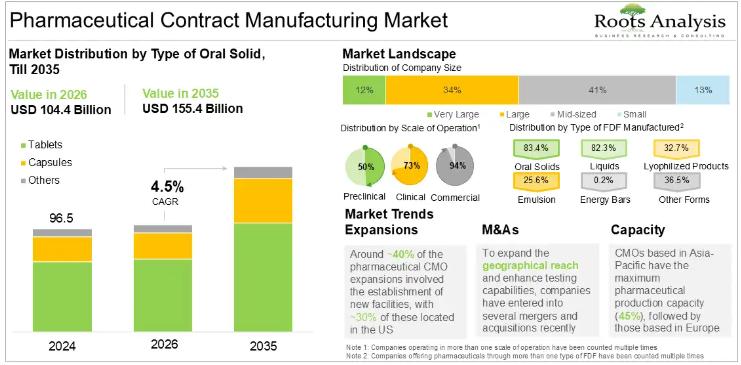
Pharmaceutical Contract Manufacturing Market CAGR To Reach 4.5% between 2025 and …
According to our latest market report "Pharmaceutical Contract Manufacturing Market by Type of Product Manufactured, Type of API, API Potency, Type of FDF, Dosage Form, Type of Oral Solid, Type of Packaging Offered, Scale of Operation, End User, Geographical Regions and Key Players: Industry Trends and Global Forecasts, till 2035", the pharmaceutical contract manufacturing market is estimated to be USD 100.3 billion in 2025. It is expected to reach USD…
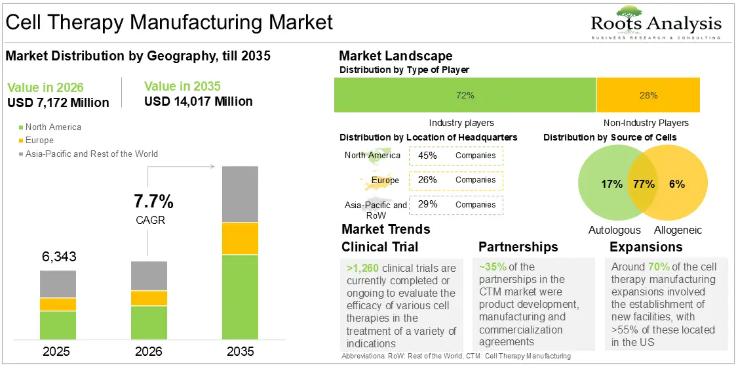
Cell Therapy Manufacturing Market CAGR To Exceed 8.25% by 2035, Due to the Growi …
According to our latest market report "Cell Therapy Manufacturing Market by Type of Cell Therapy, Source of Cells, Scale of Operation, Type of Manufacturer and Key Geographical Regions: Industry Trends and Global Forecasts, 2023-2035", the global cell therapy manufacturing market size is projected to reach USD 14,017 million by 2035 from USD 6,343 million in 2025, growing at a CAGR of 8.25% in the forecast period 2025-2035.
To request quote…
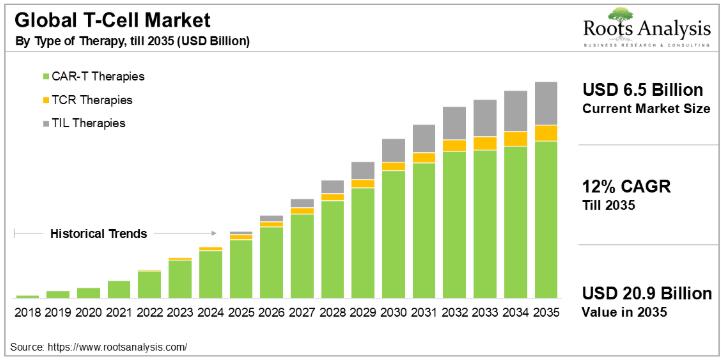
T-Cell Therapy Market Size to Hit USD 20.9 billion by 2035| Exclusive Report by …
Cancer is one of the leading causes of mortality across the world. As per the International Agency for Research on Cancer (IARC), by 2040, there are likely to be 27.5 million new cases and 16.3 million deaths related to cancer, annually. Although cancer therapeutics continue to be one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. In fact,…
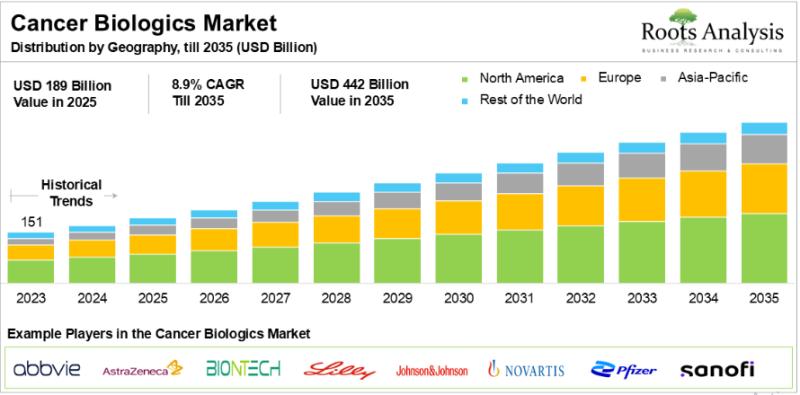
Cancer Biologics Market: Unmet Need and Treatment Guidelines
Owing to the increasing mortality rates and growing need for novel modalities to treat oncological disorders, several researchers and industry stakeholders have shifted their focus on the development of safe and effective biologic therapies. Cancer biologics are the class of therapeutic agents, which primarily modulate immune responses or directly inhibits oncogenic pathways in malignancies. These therapies, such as monoclonal antibodies, specifically target tumor-activating genes, facilitate antibody-dependent cellular cytotoxicity and complement…
More Releases for Drug
Injectable Drug Delivery Market Injectable Drug Delivery Market
Leading market research firm SkyQuest Technology Group recently released a study titled ' Injectable Drug Delivery Market Global Size, Share, Growth, Industry Trends, Opportunity and Forecast 2024-2031,' This study Injectable Drug Delivery report offers a thorough analysis of the market, as well as competitor and geographical analysis and a focus on the most recent technological developments. The research study on the Injectable Drug Delivery Market extensively demonstrates existing and upcoming…
Global Advanced Drug Delivery Systems Market Size - By Product Type(Oral Drug De …
Market Overview and Report Coverage
Advanced Drug Delivery Systems (ADDS) refer to innovative technologies designed to improve the administration and efficacy of therapeutics, enhancing the way medications are delivered to targeted areas within the body. These systems aim to optimize treatment outcomes by increasing the bioavailability, reducing side effects, and facilitating controlled drug release. Employing methods such as nanoparticles, liposomes, and implantable pumps, ADDS are revolutionizing personalized medicine and expanding therapeutic…
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Pric …
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Price, and Clinical Trials Outlook 2029 Report Highlights:
* Global Antibody Drug Conjugates Market Opportunity: > 40 Billion By 2029
* Global and Regional Antibody Drug Conjugate Market Insight
* Approved Drugs Sales Insight Global and Regional, Yearly and Quarterly, 2019 -2023
* Approved Antibody Drug Conjugates - Availability, Dosage and Price Insight
* Insight On Antibody Drug Conjugates In Clinical Trials: > 550…
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…
