Press release
A Stupendous CAGR Of 13% Between 2020 to 2030 To Be Clocked By The Atrial Fibrillation Devices Market
The Atrial Fibrillation Devices Market will witness a CAGR of 13%, reaching Expand Almost 4X between 2020 to 2030. With medical IoT implying the use of wearable monitors, devices, and various integrated applications regarding healthcare needs, the healthcare vertical is bound to scale new-fangled heights in the upcoming period. This is what the healthcare vertical would all be in the next 10 years.Interventional electrophysiology devices have already had a significant impact on patients suffering from cardiac arrhythmias, and are being increasingly adopted as a permanent treatment option for atrial fibrillation. Procedures such as radiofrequency ablation, cryoablation, and cox-maze are well performed in today’s practice.
Planning Forward? Access Sample Of Atrial Fibrillation Devices Market Report! https://www.persistencemarketresearch.co/samples/31531
Recent studies have found cryoablation to be more effective than medical management, and it is widely used in medical surgical practice. The overall market is primarily driven by the growing prevalence of atrial fibrillation amongst the geriatric as well as adult population, and the adoption of minimally-invasive techniques for the permanent treatment of atrial fibrillation.
Moreover, the use of innovative techniques, advancements in technology, and favorable reimbursement scenario are some other factors responsible for propelling the growth of the atrial fibrillation devices market.
Companies covered in Atrial Fibrillation Devices Market Report
Biosensense Webster, Inc. (Johnson & Johnson)
Abbott Laboratories
Medtronic Plc
Boston Scientific Corporation
AtriCure, Inc.
Japan Lifeline Co.
MicroPort Scientific Corporation
Biomerics
CathRx
How About Revitalizing The Strategy-Oriented Funnel To Stay Ahead In The Atrial Fibrillation Devices Market? https://www.persistencemarketresearch.co/methodology/31531
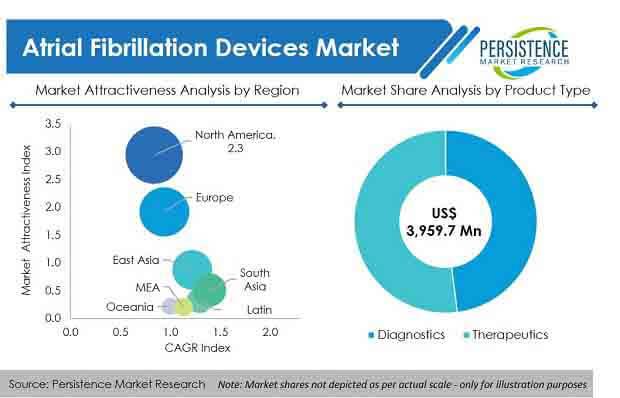
As such, the global atrial fibrillation devices market is estimated to be valued at US$ 3.9 Bn in 2020, with the market expected to exhibit a healthy CAGR of more than 14% during the forecast period (2020-2030).
Key Takeaways from Atrial Fibrillation Devices Market Study
The diagnostics segment, by product, is expected to gain maximum revenue share over the forecast period. The therapeutic segment is anticipated to increase at a CAGR of 15% over 2020–2030, to reach US$ 8.3 Bn by 2030.
According to PMR data collected from electrophysiologists in various regions, radiofrequency catheter ablation is the procedure of choice due to the high success rate, while cryoablation is the second preference for atrial fibrillation catheter ablation.
By technology, the radiofrequency and cryoablation segments, collectively held more than70% market share in terms of revenue in 2019.
Among all end users, hospitals is the leading segment, which is followed by cardiac catheterization laboratories. The hospitals segment is anticipated to expand at a CAGR of 14%over the forecast period.
North America and Europe, collectively held more than 70%revenue share in 2019, while South Asia is expected to show greater growth potential over the forecast period.
With the ongoing Covid-19pandemic wreaking havoc across the world, other non-emergency treatments have been deprioritized. As such in the near-term, the growth of the atrial fibrillation devices market will be slower than the average for the decade ahead.
“New product approvals and launches of ablation catheters along with increasing demand for minimally-invasive procedures offering increased longevity and safety profiles are driving the growth of the global atrial fibrillation devices market,” says a PMR analyst.
Planning To Introduce An Offbeat Product/Technology In The Atrial Fibrillation Devices Market? Go To “Purchase Now” To Have Our Atrial Fibrillation Devices Market Report! https://www.persistencemarketresearch.com/checkout/31531
New Product Launches – Key Strategic Focus of Market Players
Product approvals by the FDA for new AF devices are responsible for intense competition among market players. Numerous organizations are focusing on new product launches for electrophysiology, especially for atrial fibrillation.
For instance, in August 2016, Biosense Webster, Inc., a world leader in the treatment and diagnosis of heart arrhythmias, announced the launch of THERMOCOOL SMARTTOUCH SF catheters with contact force technology and a porous tip designed to optimize the treatment of atrial fibrillation.
Also, in January 2019, Abbott announced FDA approval for the TactiCath Contact Force Ablation Catheter. These winning strategies by leading players in the atrial fibrillation devices market are also being followed by regional and local players.
Atrial Fibrillation Devices Market: Competition Landscape
The global market is set to broaden its scope during the forecast period, stimulated by the rising prevalence of AF, worldwide. In addition, a favorable reimbursement scenario and increasing demand for minimally-invasive procedures have augmented the sales of atrial fibrillation devices.
The market is dominated by 4 major manufacturers – Biosense Webster, Inc. (Johnson & Johnson), Abbott Laboratories, Medtronic Plc, and Boston Scientific Corporation, with a revenue share of over 70% in 2019. These major manufacturers are focusing on manufacturing electrophysiology devices used specifically for atrial fibrillation procedure, such as ablation devices, intracardiac echocardiography catheters, and left atrial appendage (LAA) management devices.
Key market players covered by PMR include Biosense Webster, Inc. (Johnson & Johnson), Abbott Laboratories, Medtronic Plc, Boston Scientific Corporation, ArtiCure, Inc., Japan Lifeline Co., Biomerics, MicroPort Corporation, and CathRx, who are consolidating their position through mergers, acquisitions, and new product launches.
Want more insights?
PMR brings a comprehensive research report on forecasted revenue growth at global, regional, and country levels, and provides an analysis of the latest industry trends in each of the sub-segments from 2015 to 2030. The global atrial fibrillation devices market is segmented in detail to cover every aspect of the market and present a complete market intelligence approach to the reader.
The study provides compelling insights on the atrial fibrillation devices market on basis of product (diagnostics – conventional EP catheters, mapping catheters, CS catheters, and ICE catheters) and therapeutics – EP ablation, maze surgery, and LAA management devices), technology (radiofrequency, crythotherapy, ultrasound, and others), and end user (hospitals, ambulatory surgical centers, and cardiac catheterization laboratories), across seven major regions.
Access Related Reports-
Pressure Relief Devices Market @ https://www.prnewswire.com/news-releases/pressure-relief-devices-market-will-reach-at-a-cagr-of-5-5-from-2018-to-2026—persistence-market-research-300851493.html
'PMR' Related Reports-
Electrophysiology Ablation Market: https://www.persistencemarketresearch.com/market-research/electrophysiology-ablation-market.asp
Interventional Pulmonology Market: https://www.persistencemarketresearch.com/market-research/interventional-pulmonology-market.asp
Heart Valve Repair Replacement Market: https://www.persistencemarketresearch.com/market-research/heart-valve-repair-replacement-market.asp
Angiography Catheters Market: https://www.persistencemarketresearch.com/market-research/angiography-catheters-market.asp
Contact us:
Persistence Market Research
Address – 305 Broadway, 7th FloorNew York City,
NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
Website – https://www.persistencemarketresearch.com
About Us :-
Persistence Market Research is here to provide companies a one-stop solution with regards to bettering customer experience. It does engage in gathering appropriate feedback after getting through personalized customer interactions for adding value to customers’ experience by acting as the “missing” link between “customer relationships” and “business outcomes’. The best possible returns are assured therein.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release A Stupendous CAGR Of 13% Between 2020 to 2030 To Be Clocked By The Atrial Fibrillation Devices Market here
News-ID: 2429902 • Views: …
More Releases from Persistence Market Research

Dog Collars, Leashes & Harnesses Market Set to Reach US$ 9.51 Bn by 2030
Introduction
The global dog collars, leashes & harnesses market has experienced notable growth in recent years, driven by the rising adoption of companion animals, increasing pet humanization, and growing awareness of pet safety and comfort. Dogs are increasingly regarded as family members, leading pet owners to invest in high-quality, durable, and aesthetically appealing accessories that enhance both functionality and style. Collars, leashes, and harnesses play a critical role in pet control,…

Music Tourism Market Analysis Highlights 5.5% CAGR Through 2032
The global music tourism market is set to witness consistent growth over the next decade, supported by the rising popularity of live music experiences and experiential travel. According to recent industry analysis, the global music tourism market size is likely to be valued at US$9.6 billion in 2025 and is projected to reach US$14.0 billion by 2032, expanding at a compound annual growth rate (CAGR) of 5.5% during the forecast…

Electronic Dance Music Market Analysis Highlights Robust Growth Through 2031
➤ Introduction
The global Electronic Dance Music (EDM) market has emerged as one of the most dynamic and influential segments within the broader music and entertainment industry. Characterized by high-energy beats, immersive live performances, and strong digital integration, EDM has evolved from an underground cultural movement into a mainstream global phenomenon. The genre's rapid adoption across music festivals, nightclubs, streaming platforms, and social media channels has significantly reshaped consumer listening habits…

Global Memory Market Set for Strong Growth Driven by AI and Data Centers
The global memory market is entering one of its most transformative growth cycles in decades. As digital ecosystems scale rapidly across artificial intelligence (AI), cloud computing, data centers, automotive electronics, and edge devices, memory technologies are becoming the backbone of modern computing infrastructure. From high-performance servers to connected vehicles and IoT endpoints, memory capacity, speed, and efficiency now directly influence system performance and competitiveness.
The global memory market size is likely…
More Releases for Devices
Spinal Fusion Devices Market Size to Reach Valuation of $7.43 Billion by 2022 | …
Increase in adoption of minimally invasive spine surgery (MISS) presents lucrative opportunities for key players in the spinal fusion devices market. MISS is preferred to conventional techniques, owing to its associated benefits such as minimal cut or incision, which in turn reduces the chances of damage caused to the adjacent muscles.
Spinal fusion devices market was valued at $5,867 million in 2015, and is projected to reach $7,435 million by 2022,…
Global Beauty Devices Market Industry Insights Forecast to 2024, Coverage Cellul …
The global beauty devices market was valued at USD 39.1 billion in 2018 and is anticipated to grow at a CAGR of 18.4% during the forecast period. The significant growth in the beauty devices industry is imputed to the rise in prevalence of skin disorders, increasing rate of hormonal imbalance cases, an increase in the geriatric population, and growing awareness for beauty devices.
Request for Free Sample Copy of this Research…
Canada Anesthesia and Respiratory Devices Market Segments Including Airway Manag …
ReportsnReports added a new report on The Canada Anesthesia and Respiratory Devices Market report that delivers the clean elaborated structure of the Report comprising each and every business-related information of the market at a global level. The in-depth study on the current state which focuses on the major drivers and restraints for the key players. Canada Anesthesia and Respiratory Devices Market Industry research report provides granular analysis of the market…
Global Beauty Devices Market Insights 2018 By Products Hair Growth Devices,Acne …
Description
This report studies the global market size of Beauty Devices in key regions like North America, Europe, Asia Pacific, Central & South America and Middle East & Africa, focuses on the consumption of Beauty Devices in these regions.
This research report categorizes the global Beauty Devices market by players/brands, region, type and application. This report also studies the global market status, competition landscape, market share, growth rate, future trends, market drivers,…
Wearable Electronic Devices Market,Wearable Electronic Devices Industry, Global …
Latest industry research report on: Global Wearable Electronic Devices Market : Industry Size, Share, Research, Reviews, Analysis, Strategies, Demand, Growth, Segmentation, Parameters, Forecasts
This report studies the global Wearable Electronic Devices market status and forecast, categorizes the global Wearable Electronic Devices market size (value & volume) by manufacturers, type, application, and region. This report focuses on the top manufacturers in United States, Europe, China, Japan, South Korea and Taiwan and other…
Ophthalmic Devices Market By Product Function [Ophthalmic Surgery Devices (Refra …
Ophthalmology is a branch of medical sciences that deals with the structure, function, and various eye diseases. The ophthalmic devices are medical equipment designed for diagnosis, surgical, and vision correction purposes. These devices gain increased importance and adoption due to high prevalence of various ophthalmic diseases such as glaucoma, cataract, and other vision related issues.
Request Sample At: https://www.bigmarketresearch.com/request-sample/1074435
Increase in prevalence rate of eye related diseases such as glaucoma, cataract,…