Press release
North America Medical Device Regulatory Affairs Outsourcing Market | Parexel International Corporation, North American Science Associates, Inc., SGS SA, Creganna
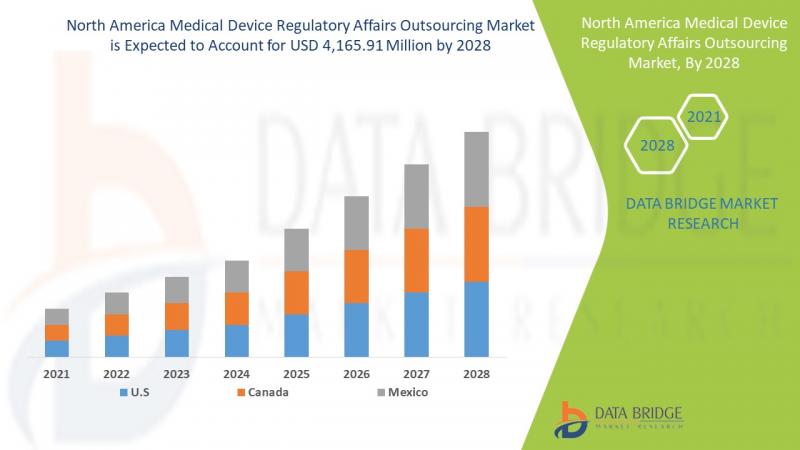
The medical device regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with a CAGR of 12.8% in the forecast period of 2021 to 2028 and is expected to reach USD 11,935.77 million by 2028. The strategic initiative for geographical expansions is anticipated to drive the growth of the medical device regulatory affairs outsourcing marketDownload Free Sample Copy @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=north-america-medical-device-regulatory-affairs-outsourcing-market
Outsourcing is an important part of every pharmaceutical and biotechnology companies’ value chain during research and development (R&D). The regulatory affairs outsourcing services entail medical writing and publication of regulatory documentation by professional medical authors, quality control (QC) auditors and publishers who contribute to high-quality clinical research projects. The demand for regulatory services outsourcing has been fueled by a substantial increase in clinical studies conducted in emerging economies, providing a healthy platform for this industry's growth.
The increasing number of patent expirations acts as driver for its growth in the medical device regulatory affairs outsourcing market. The fluctuation in the prices of various medical devices regulatory affairs services acts as restraint for its growth in the medical device regulatory affairs outsourcing market. The awards and recognition provides excellent opportunity for the medical device regulatory affairs outsourcing market growth. The pandemic outbreak of COVID-19 acts as challenge for the growth of the medical device regulatory affairs outsourcing market.
Request for TOC @ https://www.databridgemarketresearch.com/toc/?dbmr=north-america-medical-device-regulatory-affairs-outsourcing-market
Medical Device Regulatory Affairs Outsourcing Market Scope and Market Size
The medical device regulatory affairs outsourcing market is segmented on the based on the basis of services, product, device type, application and end user. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
• On the basis of services, the medical device regulatory affairs outsourcing market is segmented into regulatory affairs services, quality consulting and medical writing. In 2021, the regulatory affairs services segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing by key medical device companies.
• On the basis of product, the medical device regulatory affairs outsourcing market is segmented into finished goods, electronics and raw material. In 2021, the finished goods segment is expected to dominate the medical device regulatory affairs outsourcing market due to the increased adoption of regulatory affairs outsourcing for the finished goods by major medical device companies.
• On the basis of device type, the medical device regulatory affairs outsourcing market is segmented into class I, class II and class III. In 2021, the class I segment is expected to dominate the medical device regulatory affairs outsourcing market because of the rising demand for medical devices worldwide to treat patients with chronic diseases.
• On the basis of application, the medical device regulatory affairs outsourcing market is segmented into cardiology, diagnostic imaging, orthopedic, IVD, ophthalmic, general and plastic surgery, drug delivery, dental, endoscopy, diabetes care and others. In 2021, the cardiology segment is expected to dominate the medical device regulatory affairs outsourcing market because of the increased adoption of regulatory affairs outsourcing for the class III medical devices by key medical device companies.
• On the basis of end user, the medical device regulatory affairs outsourcing market is segmented into small medical device company, medium medical device company and large medical device company. In 2021, the medium medical device company segment is expected to dominate the medical device regulatory affairs outsourcing market due to the rising demand for medical devices worldwide.
Inquiry Before Buy @ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=north-america-medical-device-regulatory-affairs-outsourcing-market
Medical Device Regulatory Affairs Outsourcing Market Country Level Analysis
Medical device regulatory affairs outsourcing market is analyzed and market size information is provided by the country, services, product, device type, application and end user as referenced above.
The countries covered in the medical device regulatory affairs outsourcing market report are the Japan, China, Australia, India, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific.
China is the leading country in the growth of the Asia-Pacific medical device regulatory affairs outsourcing market due to growing R&D activities for the regulatory affairs services segment.
Competitive Landscape and Medical Device Regulatory Affairs Outsourcing Market Share Analysis
The medical device regulatory affairs outsourcing market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus related to the Asia-Pacific medical device regulatory affairs outsourcing market.
The major companies covered in the Asia-Pacific medical device regulatory affairs outsourcing market report are Parexel International Corporation, North American Science Associates, Inc., SGS SA, Creganna (a subsidiary of TE Connectivity), Intertek Group plc, WuXi AppTec, Charles River Laboratories, Celestica Inc., Freyr, Cactus Communications, Cekindo Business International, Eurofins Scientific, TÜV SÜD, Sterigenics U.S., LLC – A Sotera Health company, TE Connectivity, FLEX LTD., Heraeus Holding, Integer Holdings Corporation, Nortech Systems, Inc., IQVIA, Covance, Plexus Corp., Sanmina Corporation, OMICS International, East West Manufacturing, Jabil Inc., Omron Corporation among other global and domestic players. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Related Reports:
https://www.databridgemarketresearch.com/reports/global-alad-porphyria-treatment-market
https://www.databridgemarketresearch.com/reports/global-chromatography-consumables-market
https://www.databridgemarketresearch.com/reports/global-large-volume-parenteral-market
https://www.databridgemarketresearch.com/reports/global-light-emitting-diode-led-probing-and-testing-equipment-market
Contact Us:-
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email us:-sopan.gedam@databridgemarketresearch.in
About Data Bridge Market Research Private Ltd:
Data Bridge Market Research Pvt Ltd is a multinational management consulting firm with offices in India and Canada. As an innovative and neoteric market analysis and advisory company with unmatched durability level and advanced approaches. We are committed to uncover the best consumer prospects and to foster useful knowledge for your company to succeed in the market.
Data Bridge Market Research is a result of sheer wisdom and practice that was conceived and built-in Pune in the year 2015. The company came into existence from the healthcare department with far fewer employees intending to cover the whole market
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release North America Medical Device Regulatory Affairs Outsourcing Market | Parexel International Corporation, North American Science Associates, Inc., SGS SA, Creganna here
News-ID: 2401609 • Views: …
More Releases from databridgemarketresearch

Medical Electronics Market to Exhibit a Remarkable CAGR of 8% by 2029, Size, Sha …
The medical electronics market is expected to witness market growth at a rate of 8% in the forecast period of 2022 to 2029.
Market Definition:
Medical electronics is referred to as a particular discipline that assimilates engineering with fields including clinical practice and biomedical sciences. Unlike paper records, medical electronics provides more advantages. Further allowing an individual track information over time it enhances the quality of patient care.
Download Sample PDF Copy…

Surgical Sponges Market to Exhibit a Remarkable CAGR of 3.60% by 2029, Size, Sha …
Data Bridge Market Research analyses that the surgical sponges market which was USD 2221.91 million in 2021, would rocket up to USD 2948.52 million by 2029, and is expected to undergo a CAGR of 3.60% during the forecast period 2022 to 2029.
Market Definition:
A surgical sponge is a specific type of cotton pad that is employed in surgeries to absorb blood and other bodily fluid flow. These sponges are useful for…

Antifibrinolytic Market to Exhibit a Remarkable CAGR of 5% by 2029, Size, Share, …
Data Bridge Market Research analyses a growth rate in the global antifibrinolytic market in the forecast period 2022-2029. The expected CAGR of global antifibrinolytic market is tend to be around 5% in the mentioned forecast period.
Market Definition:
Antifibrinolytic are a type of therapeutics that helps in blood clotting by restricting the process called fibrinolysis. Many antifibrinolytics such as tranexamic acid exert their action by reversibly binding to the lysine receptor…

Oxygen Delivery Systems Market to Exhibit a Remarkable CAGR of 6.3% by 2029, Siz …
Data Bridge Market Research analyses that the oxygen delivery systems market which was USD 12,949.38 million in 2021, is expected to reach USD 21111.31 million by 2029, at a CAGR of 6.3% during the forecast period 2022 to 2029.
Market Definition:
A device used to help, regulate, and supplement oxygen to patients in order to increase arterial oxygenation is known as an oxygen delivery system. Oxygen delivery systems provide oxygen therapy, which…
More Releases for Medical
ECG Analysis System Market Research Report 2022 - GE Medical, Medical Econet, Gr …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global ECG Analysis System. On the basis of historic growth analysis and current scenario of ECG Analysis System place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
ECG Analysis System Market Outlook: 2020 The Year On A Positive Note | GE Medica …
DataIntelo report titled Global ECG Analysis System Market provides detailed information and overview about the key influential factors required to make well informed business decision. This is a latest report, covering the current COVID-19 impact on the market. The pandemic of Coronavirus (COVID-19) has affected every aspect of life globally. This has brought along several changes in market conditions. The rapidly changing market scenario and initial and future assessment of…
Medical Tracheostomy Tube Market Overall Study Report 2020-2027 | Players Medtro …
The latest report added by Stratagem Market Insights gives deep insights into the drivers and restraints in the Worldwide Medical Tracheostomy Tube Market. The research report "Global Medical Tracheostomy Tube Market Size and Growth Forecast to 2027" provide a comprehensive take on the overall market. Analysts have carefully evaluated the milestones achieved by the global Medical Tracheostomy Tube market and the current trends that are likely to shape its future.…
Global Embolization Particle Market 2019 - Sirtex Medical, Merit Medical, Cook M …
The global "Embolization Particle Market" report delivers a comprehensive and systematic framework of the Embolization Particle market at a global level that includes all the key aspects related to it. The data is collected from different sources allied to the global Embolization Particle market and the research team meticulously analyze the gathered data with the help of various analytical tools and present their opinion based on analysis and calculations. The…
Medical Casting & Splinting Market 2019: Top Key players are 3M, DJO Global, BSN …
Medical Casting & Splinting Market 2019 Report analyses the industry status, size, share, trends, growth opportunity, competition landscape and forecast to 2025. This report also provides data on patterns, improvements, target business sectors, limits and advancements. Furthermore, this research report categorizes the market by companies, region, type and end-use industry.
Get Sample Copy of this Report@ https://www.researchreportsworld.com/enquiry/request-sample/13718924
Global Medical Casting & Splinting market 2019 research provides a basic overview of…
Global Oxygen Pressure Regulator Market 2017 : Precision Medical, Smiths Medical …
Oxygen Pressure Regulator
A market study based on the " Oxygen Pressure Regulator Market " across the globe, recently added to the repository of Market Research, is titled ‘Global Oxygen Pressure Regulator Market 2017’. The research report analyses the historical as well as present performance of the worldwide Oxygen Pressure Regulator industry, and makes predictions on the future status of Oxygen Pressure Regulator market on the basis of this analysis.
Get Free…