Press release
In Vitro Diagnostics (IVD) Quality Control Market Technology, Source, Manufacturer, End Users and Top Companies - Bio-Rad Laboratories, Inc., Randox Laboratories Ltd., Thermo Fisher Scientific, Inc., LGC Limited, Abbott Laboratories
The In Vitro Diagnostics (IVD) Quality Control Market report proposes the forecast of the market by studying the market dynamics of the previous years. The report proposes the estimated market size and the expected global revenue for the forecast period of the Medical Devices industry. It is the result of a detailed analysis of the market status, demands, growth factors, market drivers, growth rate, opportunities and limitations, risks, sales data, profit margin, channels, and distributors. The main motive of this In Vitro Diagnostics (IVD) Quality Control Market report is to derive the revenue growth, market value, market share, etc., by studying the categories such as the prime market players from different geographical locations, their product types, and application industries.Get a FREE Sample Copy of this Report with Graphs and Charts at: https://www.reportsnreports.com/contacts/requestsample.aspx?name=515130
The global IVD quality control market is projected to reach USD 1.4 billion by 2026 from USD 1.1 billion in 2021, at a CAGR of 5.3% during the forecast period.
Key players in the IVD quality control Market
Some of the key players in the market include Bio-Rad Laboratories, Inc. (US), Randox Laboratories Ltd. (UK), Thermo Fisher Scientific, Inc. (US), LGC Limited (UK), and Abbott Laboratories (US). Other prominent payers in the market include Roche Diagnostics (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), Fortress Diagnostics (UK), SERO AS (US), Sysmex Corporation (Japan), Ortho-Clinical Diagnostics (US), Helena Laboratories Corporation (US), Quidel Corporation (US), Sun Diagnostics, LLC (US), Seegene Inc. (South Korea), ZeptoMetrix Corporation (US), Qnostics (UK), Bio-Techne Corporation (US), Microbiologics (US), Microbix Biosystems (Canada), Streck, Inc. (US), Alpha-Tec Systems (US), Maine Molecular Quality Controls, Inc. (US), and Grifols, S.A. (Spain).
The break of primary participants was as mentioned below:
By Company Type – Tier 1–60%, Tier 2–30%, and Tier 3–10%
By Designation – C-level–30%, Director-level–50%, Others–20%
By Region – North America–45%, Europe–15%, Asia Pacific–25%, Latin America- 10%, Middle East and Africa–5%
The product & service segment holds the highest share of the total IVD quality control market during the forecast period.
Based on product & service, the IVD quality control market is segmented into quality control products, data management solutions, and quality assurance services. The quality control products segment accounted for the largest share of the IVD quality control market in 2020. The increasing number of accredited laboratories and mandates for the use of quality controls from regulatory bodies to ensure the accuracy of diagnostic test results are driving the growth of the IVD quality control products market.
Serum based controls drive the growth of the quality control products segment during the forecast period.
Based on type, the IVD quality control products market is segmented into serum/plasma-based controls, whole blood-based controls, urine-based controls, and others. Serum/plasma-based controls are highly preferred by laboratories; this segment accounted for the largest share of the quality control products market in 2020. The greater uptake of serum/plasma-based quality controls among laboratories and the wide application areas of these controls for various IVD tests are driving the market growth
Molecular diagnostics segment accounted for the highest CAGR of the global IVD quality control market
Based on technology, the IVD quality control market is broadly segmented into clinical chemistry, immunochemistry, hematology, coagulation & hemostasis, microbiology, molecular diagnostics, and other technologies. The molecular diagnostics segment has the highest CAGR for the forecast period. With the increased non-communicable disease burden, molecular diagnostic tests have been used in the field of oncology widely. Technological advancement in the field of molecular diagnostics is another driver for the growth of this industry.
Third-party controls accounted for the highest share for the IVD quality control market
Based on manufacturer, the IVD quality control market is segmented into third-party controls and OEM controls. The third-party controls segment accounted for the largest share of the global IVD quality control market in 2020. The large share of this segment can be attributed to the increasing use of third-party quality controls across the globe to verify the accuracy and reliability of tests
Based on end users, hospitals accounted for the highest share of the global IVD quality control market during the forecast period.
The key end users of IVD quality controls studied in this report include hospitals, clinical laboratories, research & academic institutes, and other end users. The hospitals segment accounted for the largest share of the IVD quality control market in 2020, owing to the large volume of diagnostic tests carried out in hospitals.
North America is expected to account for the highest share for players operating in the global IVD quality control market
Geographically, the global IVD quality control market studied in this report is divided into five major regions—North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. In 2020, North America accounted for the largest share of the global IVD quality control market, followed by Europe. The APAC region has the highest CAGR of the global IVD quality control market. The Asia Pacific is considered the most lucrative market for IVD quality controls, owing to the region’s large patient population and rising healthcare needs. Market players are focusing on improving laboratory test quality in emerging APAC countries by working with local government bodies, agencies, and academic societies to offer external QC for standardizing laboratory testing procedures and ensure accuracy.
Reasons to Buy the Report
The report will enrich established firms as well as new entrants/smaller firms to gauge the pulse of the market, which in turn would help them, garner a more significant share of the market. Firms purchasing the report could use one or any combination of the below-mentioned strategies to strengthen their position in the market.
This report provides insights into the following pointers:
Market Penetration: Comprehensive information on product portfolios offered by the top players in the global IVD quality control market. The report analyzes this market by product, technology, manufacturer type, end user, and region.
Product Enhancement/Innovation: Detailed insights on upcoming trends and product launches in the global IVD quality control market
Market Development: Comprehensive information on the lucrative emerging markets by product, technology, manufacturer type, end user, and region.
Market Diversification: Exhaustive information about new products or product enhancements, growing geographies, recent developments, and investments in the global IVD quality control market
Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings, competitive leadership mapping, and capabilities of leading players in the global IVD quality control market.
Get 20% Discount on this Research Report at: https://www.reportsnreports.com/contacts/discount.aspx?name=515130
Table Of Contents
1 Introduction
1.1 Objectives Of The Study
1.2 Market Definition
1.2.1 Inclusions & Exclusions Of The Study
1.3 Market Scope
1.3.1 Markets Covered
1.3.2 Years Considered For The Study
1.3.3 Currency
Table 1 Exchange Rates Utilized For Conversion To Usd
1.4 Stakeholders
1.5 Summary Of Changes
2 Research Methodology
2.1 Research Data
Figure 1 Research Design
2.1.1 Secondary Data
2.1.1.1 Key Data From Secondary Sources
2.1.2 Primary Data
Figure 2 Primary Sources
2.1.2.1 Key Data From Primary Sources
2.1.2.2 Key Industry Insights
Figure 3 Breakdown Of Primary Interviews: By Company Type, Designation, And Region
Figure 4 Breakdown Of Primary Interviews: Supply-Side And Demand- Side Participants
2.2 Market Size Estimation
Figure 5 Supply-Side Market Size Estimation: Revenue Share Analysis
Figure 6 Revenue Share Analysis Illustration: Bio-Rad Laboratories, Inc.
Figure 7 Revenue Analysis Of The Top Five Companies: IVD Quality Control Market (2020)
Figure 8 Cagr Projections From The Analysis Of Drivers, Restraints, Opportunities, And Challenges Of The IVD Quality Control Market (2021–2026)
Figure 9 Cagr Projections
Figure 10 Top-Down Approach
2.3 Market Breakdown & Data Triangulation
Figure 11 Market Data Triangulation Methodology
2.4 Market Share Analysis
2.5 Assumptions For The Study
2.6 Risk Assessment
Table 2 Risk Assessment: IVD Quality Control Market
2.7 Limitations
2.7.1 Methodology-Related Limitations
2.7.2 Scope-Related Limitations
3 Executive Summary
Figure 12 IVD Quality Control Market, By Product & Service, 2021 Vs. 2026 (Usd Million)
Figure 13 IVD Quality Control Products Market, By Type, 2021 Vs. 2026 (Usd Million)
Figure 14 IVD Quality Control Market, By Manufacturer, 2021 Vs. 2026 (Usd Million)
Figure 15 Third-Party Controls Market, By Type, 2021 Vs. 2026 (Usd Million)
Figure 16 IVD Quality Control Market, By Technology, 2021 Vs. 2026 (Usd Million)
Figure 17 IVD Quality Control Market, By End User, 2021 Vs. 2026 (Usd Million)
Figure 18 IVD Quality Control Market: Geographic Snapshot
4 Premium Insights
4.1 IVD Quality Control Market Overview
Figure 19 Growing Adoption Of Third-Party Controls And Increasing Demand For External Quality Assessment To Drive Market Growth
4.2 Apac: IVD Quality Control Market, By Technology And Country (2020)
Figure 20 Immunochemistry To Command The Largest Share Of The Apac IVD Quality Control Market In 2020
4.3 IVD Quality Control Market: Geographic Mix
Figure 21 China To Grow At The Highest Cagr During The Forecast Period
4.4 IVD Quality Control Market: Developed Vs Developing Markets
Figure 22 Developing Markets To Register Highest Cagr During The Forecast Period
5 Market Overview
6 IVD Quality Control Market, By Product And Service
7 IVD Quality Control Market, By Technology
8 IVD Quality Control Market, By Manufacturer
Purchase this report at: https://www.reportsnreports.com/purchase.aspx?name=515130
Contact Us:
Corporate Headquarters
Tower B5, office 101,
Magarpatta SEZ,
Hadapsar, Pune-411013, India
+ 1 888 391 5441
sales@reportsandreports.com
About Us:
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets. With comprehensive information about the publishers and the industries for which they publish market research reports, we help you in your purchase decision by mapping your information needs with our huge collection of reports.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In Vitro Diagnostics (IVD) Quality Control Market Technology, Source, Manufacturer, End Users and Top Companies - Bio-Rad Laboratories, Inc., Randox Laboratories Ltd., Thermo Fisher Scientific, Inc., LGC Limited, Abbott Laboratories here
News-ID: 2385522 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
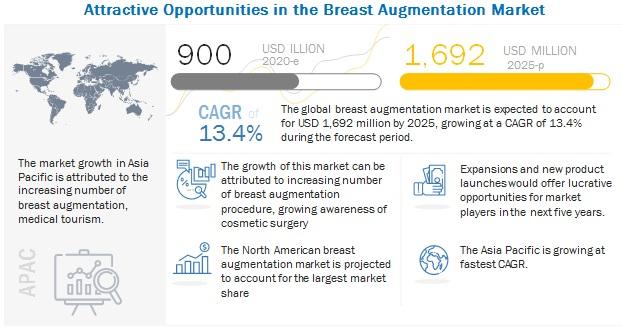
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…