Press release
Biosimilars Market to Reach USD 16.33 Billion by 2030 | Top Players Covered are Novartis AG (Switzerland), Mylan N.V. (U.S), Coherus BioSciences, Inc. (U.S.), AbbVie Inc. (U.S/), Pfizer Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Eli Lilly and C
The global biosimilars market size was USD 10.25 billion in 2020. The market is expected to grow from USD 11.40 billion in 2021 to USD 16.33 billion by 2030 at a CAGR of 24.42% in the 2021-2030 period. This information is published by “Decision Foresight”, in its report, titled, “Biosimilars Market, 2021-2030.”A biosimilar, also known as a reference biological product, is a biological product that is extremely similar to a previously FDA-approved medication. Biosimilars are medications that have been authorised by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) and have no clinically significant differences in terms of safety, purity, efficacy, or effectiveness when compared to the reference product. Biosimilar medications can only be authorised for indications and conditions that have already been approved for reference product by major regulatory bodies.
The FDA extended the biosimilar category to include 90 more compounds in March 2020, meaning there are now more treatments that potentially serve as biosimilar reference products. This is crucial because it allows biosimilar research across a variety of therapeutic areas, resulting in increasing competition. Some of these markets haven't seen many new entrants or more inexpensive care alternatives in a long time, which may result in a major shake-up and lower healthcare expenses. This is expected to amplify the demand and promote the Biosimilars market growth during the forecast period.
Request a Sample Report:
https://www.decisionforesight.com/request-sample/DFS020175
List of Key Players Covered in this Market Report are:
• Novartis AG (Switzerland)
• Mylan N.V. (U.S)
• Coherus BioSciences, Inc. (U.S.)
• AbbVie Inc. (U.S/)
• Pfizer Inc. (U.S.)
• F. Hoffmann-La Roche Ltd. (Switzerland)
• Eli Lilly and Company (U.S.)
• Teva Pharmaceutical Industries Ltd. (Israel)
• Celltrion Inc. (South Korea)
• Amgen Inc. (U.S)
COVID-19 Impact: Rising Demand for the Innovative Drugs with Surge in FDA Approvals Observed amid Pandemic
COVID-19 may have a big influence on the biosimilars industry, and it has provided a substantial barrier to pharmaceutical firms working on biosimilar development. During the current pandemic, the FDA's approval of non-COVID medicines has been reduced, which is likely to slow the process of product approval and marketing, stifling market growth.
Furthermore, because most clinical studies have been postponed in order to battle the COVID-19 issue and reduce the risk of infection among participants, most pipeline items are moving at a snail's pace in terms of research and development. There is also a supply scarcity owing to the worldwide lockdown and travel restrictions.
Segmentation
On the basis of drug class filgrastim and pegfilgrastim, monoclonal antibodies, and others are the medication classes that make up the market. Filgrastim and pegfilgrastim are dominating the market, due to the USFDA's approval and introduction of these products. The involvement of major pharmaceutical companies such as Mylan N.V. and Pfizer, Inc. in the development and marketing of filgrastim and pegfilgrastim in the United States is expected to fuel sector expansion in the future years.
Based on disease indication, market is segmented between cancer, autoimmune disorders, and others. Autoimmune illness has the largest market share. Increased medication approvals and the launch of additional biosimilars for neutropenia and arthritis in the United States, as well as excellent success of brands like Renflexis, Zarxio, and Inflectra, have contributed to the domination. During the projection period, cancer is expected to have the second biggest market share.
Based on distribution channels, hospital pharmacies, retail pharmacies, and internet pharmacies are the three types. In terms of revenue, hospital pharmacies led the market and are expected to continue to do so during the projected period.
Browse Summary of This Research Report:
https://www.decisionforesight.com/reports/biosimilars-market
Drivers and Restraints
Expiring Patents of Biological Drugs, Initiatives for financial sustainability and cost-cutting and Growing Geriatric Population to Drive Market Growth Factors such as the increased prevalence of chronic illnesses such as cancer and diabetes, as well as the growing demand for pharmaceutical medications, particularly high-priced patented pharmaceuticals, are driving the industry. The high cost of reference goods, on the other hand, limits market expansion by increasing the financial burden on patients and reimbursement service providers. A factor that contributes to these high prices is a lack of economies of scale due to reduced demand.
Furthermore, the absence of regulatory standards, consumer brand preferences, physician reluctance to prescribe biosimilars, and the expensive capital necessary for research and development are all impeding the growth of the biosimilars industry.
Expiring patents on several biological drugs, initiatives for financial sustainability and cost-cutting, growing geriatric population are the main driving factors of this market. Many biological medication patents have expired throughout the years, and many more will expire in the coming years, raising the need for Biosimilar Medicines. The primary goal of biosimilar medicines is to make vital medications more accessible to everyone. In many nations, the government is taking steps to promote biosimilar medications. As the population of the elderly grows, so does the number of persons who are susceptible to chronic illnesses. As a result, biosimilar medications are in increasing demand.
Regional Insights
The biosimilars market in North America is expected to grow at a substantial CAGR over the projected period. The increasing prevalence of chronic illnesses, such as cancers, as well as increased investment in research and development activities by the key players, are the main drivers driving the expansion of the studied market in the area.
According to Globocon 2020, in 2020, there will be 2,281,658 new cancer cases diagnosed in the United States, with 612,390 fatalities. Breast cancer had the greatest incidence of all malignancies, with 253,465 cases, followed by lung (227,875), prostate (209,512), and colon cancer (101,809).
Furthermore, according to a study published in Biosimilar Development 2020, the majority of biosimilars are injectables that must be administered to patients by a clinician. Due of the high prevalence of SARS-CoV2 viral transmission, most hospitals and clinics in the United States have prohibited physical consultations. During the pandemic, this factor is likely to stifle market growth in the region. In addition, North America is home to a number of major market participants, including Pfizer Inc., Mylan NV, Amgen Inc., and Coherus Biosciences Inc., among others.
Competitive Landscape
Advanced and innovative Tactics Adopted by Key Players to Sustain their Position
In terms of revenue, the market has consolidated, with two companies accounting for more than two third of the market. Due to higher sales of Zarxio and Erelzi, Novartis AG is expected to maintain its dominance in the US biosimilars market. Pfizer Inc.'s market position in the US biosimilars market is projected to grow as a result of FDA approvals for TRAZIMERA and NIVESTYM, as well as a strong pipeline of medicines. Merck & Co., F. Hoffmann-La Roche Ltd., Coherus BioSciences, Inc., AbbVie Inc., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Celltrion Inc., and others are among the market's other competitors.
Inquire Before Buy:
https://www.decisionforesight.com/inquire/DFS020175
Industry Development
June 2020: Pfizer Inc. received the FDA Clearance for its pegfilgrastim biosimilar, Nyvepria, which is indicated for use in lowering the probability of infections.
Contact Us:
Phone: +919875577842
Email: sales@decisionforesight.com
About Us:
Decision Foresight is a market research organization known for its reliable and genuine content, market estimation and the best analysis which is designed to deliver state-of-the-art quality syndicate reports to our customers. Apart from syndicate reports, you will find the best market insights, strategies that will help in taking better business decisions on subjects that may require you to develop and grow your business-like health, science, technology and many more. At Decision Foresight, we truly believe in disseminating the right piece of knowledge to a large section of the audience and cover the in-depth insights of market leaders across various verticals and horizontals.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars Market to Reach USD 16.33 Billion by 2030 | Top Players Covered are Novartis AG (Switzerland), Mylan N.V. (U.S), Coherus BioSciences, Inc. (U.S.), AbbVie Inc. (U.S/), Pfizer Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Eli Lilly and C here
News-ID: 2348944 • Views: …
More Releases from Decisionforesight

Mobile Advertising Market Size, Share and Forecast 2030 | Tencent's Digital Ad R …
The global mobile advertising market size was valued at USD 76.9 billion in 2020 and it is expected to reach USD 1234.5 billion by 2030 with the CAGR of 32% during 2020-2030. This information is published by “Decision foresight“, in its report, titled, “Global Mobile Advertising Market, 2020-2030.”As a result of the growth in mobile usage, mobile app advertising expenditure has skyrocketed.
According to the latest data from the Interactive Advertising…

Energy Drinks Market Size Forecast 2030 | The increased popularity of alternativ …
According to the research study, The global energy drinks market size was valued at USD 60.8 billion in 2020 and is anticipated to reach USD 120.7 billion by 2030 with a CAGR of 7.10% over the forecast period. in the research report, titled, “Global Energy Drinks Market, 2020-2030.”Carbonated beverages, fruit and vegetable juices, bottled water, sports drinks, beverage concentrates, ready-to-drink tea, and ready-to-drink coffee are all included in the soft drink…

Cybersecurity Market Size, Share and Forecast 2030 | Introduction of Disruptive …
The global cybersecurity market held USD 157 billion in 2020 and is to grow with a CAGR of 8.89% from 2021-2030. This information is published by “Decision foresight”, in its report, titled, “Global Cybersecurity Market, 2020-2030.”The data and integrity of computing resources connected to or placed on a business network are protected by Cybersecurity. Its goal is to protect these resources from all types of hackers throughout the assault cycle.
One…
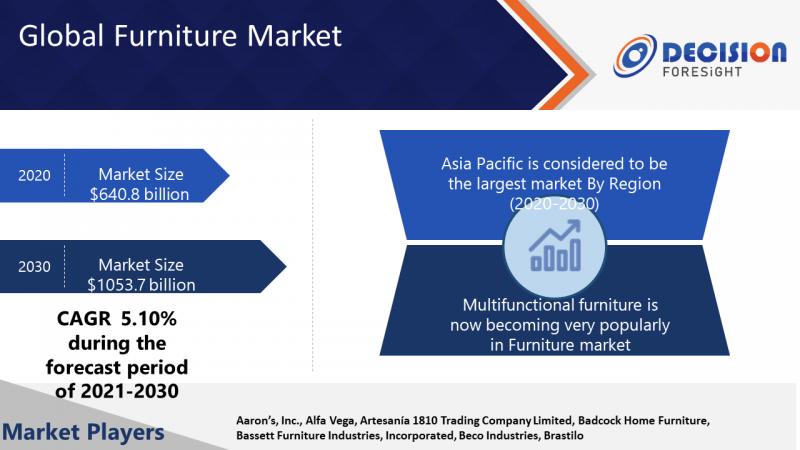
Furniture Market Size Was Valued at USD 640.8 Billion in 2020 and Is Anticipated …
The global furniture market size was valued at USD 640.8 billion in 2020 and is anticipated to reach USD 1053.7 billion by 2030 with a CAGR of 5.10% over the forecast period. This information is published by “Decision foresight”, in its report, titled, “Global Furniture Market, 2020-2030.”With a growing worldwide population and a growing middle class in almost every part of the globe, demand for house furnishings is increasing, allowing…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…