Press release
Global Regenerative Medicine Market Key Players Analysis Ocata Therapeutics, Athersys, Baxter International, Caladrius Biosciences, Cynata Therapeutics, Cytori Therapeutics, MEDIPOST, Mesoblast
This report provides a comprehensive overview of the size of the regenerative medicine market, segmentation of the market (stem cells, tissue engineering and CAR-T therapy), key players and the vast potential of therapies that are in clinical trials. Kelly Scientific analysis indicates that the global regenerative medicine market was worth $35 billion in 2019 and will grow to over $124 billion by 2025, with a CAGR of 23.3% between this time frame. Within this market, the stem cell industry will grow significantly at a CAGR of over x% and reach $x billion by 2025. Tissue engineering is forecast to grow at a CAGR of x% to 2025 and potentially reach $x billion. This report describes the evolution of such a huge market in 15 chapters supported by over 350 tables and figures in 700 pages.Get a Free Sample Copy of Global Regenerative Medicine Market Research Report at https://www.reportsnreports.com/contacts/requestsample.aspx?name=4705521
• An overview of regenerative medicine that includes: stem cells, allogenic and autogenic cells, umbilical cord blood banking, tissue engineering and CAR T therapies.
• Global regenerative medicine market, global breakdown, application breakdown and leading market players
• Detailed account of the stem cell industry market by geography, indication and company profiles
• Profiles, marketed/pipeline products, financial analysis and business strategy of the major companies in this space
• Focus on current trends, business environment, pipeline products, clinical trials, and future market forecast for regenerative medicine
• Insight into the challenges faced by stakeholders, particularly about the success vs. failure ratios in developing regenerative medicine drugs and therapies.
• Insight into the biobanking industry globally and its impact on the overall market
• Description and data for the prevalence of disease types that are addressed by regenerative medicine, stem cells, tissue engineering and CAR-T therapies
• Financial market forecast through 2023 with CAGR values of all market segments outlined in the objective
• SWOT analysis of the global market
• Geographical analysis and challenges within key topographies including the USA, Japan, South Korea, China and Europe
Countries Covered
• Global, USA, Europe, UK, Japan, South Korea, Singapore, Asia/Pacific, ROC
Regenerative medicine’s main objective is to heal and replace organs/cells that have been damaged by age, trauma or disease. Congenital defects can also be addressed with regenerative medicine. Therefore, it’s market encompasses dermal wounds, cardiovascular disease, specific cancer types and organ replacement. To that end, regenerative medicine is a broader field and manipulates the body’s immune system and regeneration potential to achieve its requirement. Financially speaking, investment into this space is dominated by grants, private investors and publicly traded stocks. Looking forward, the regenerative medicine market is promising for a number of robust reasons including:
• Increasing number of potentially successful clinical trials
• Increasing number of mergers and acquisitions
• High unmet need in many indications
• Global penetration, especially in Japan will boost the market
Of course restrictions to this market include strict regulations in certain geographies, and also the level of investment required to support R&D, clinical research, trials and commercialization. Reimbursement strategies are also paramount to success of the overall space.
Get 25% Discount on this Research Report at https://www.reportsnreports.com/contacts/discount.aspx?name=4705521
There are over 700 regenerative medicine companies globally at present, that all together have a $x billion market cap. At present the total regenerative medicine market has more than 500 products commercialized. The regenerative medicine market encompasses a number of key technology submarkets including:
• Cell therapy including stem cells
• Tissue Engineering
• Biomaterials
• BioBanking
Reconstructive surgeries for bones and joints is the mainstay of the regenerative medicine market. Geographically speaking, due to the dominance of the bone and joint reconstruction market, the US has the biggest space. This is followed by Europe. However, due to recent positive legislation in Japan and Europe, the stem cell arena will grow more substantially in these regions over the next five years. By 2025, it is possible that Europe will surpass the US market with respect to stem cell applications, and this will become more likely if the Trump Administration restricts legislation and funding.
Market Applications & Opportunities for Regenerative Therapies
Regenerative medicine, including cellular and gene therapies will have a significant impact on the expenditure of payers, once reimbursement schemes are optimized. To that end a number of conditions that regenerative medicine tackles is synonymous with an aging population such as
• Cardiovascular diseases & stroke
• Diabetes
• Inflammatory and immune diseases
• Wound healing and soft tissue regeneration
• Neurodegenerative diseases e.g., ALS, Alzheimer’s and Parkinson’s
• Spinal cord injury
• Musculoskeletal disorders
• Ocular disease
Global Financial Landscape
The last few years have been busy for regeneration medicine, cellular therapeutics and the gene therapy industry, with high investment from pharma giants such as Eli Lilly, BMS, Astra Zeneca and Sanofi. Company partnerships were also in motion that included Kite Pharma and Bluebird/Five Prime, Juno and Fate Therapeutics/ Editas Medicine. One of the highlights was the $x billion, four year deal between CRISPR Technologies and Vertex which indicated that gene editing technologies are cutting edge.
Stem Cell Market Analysis & Forecast to 2025
Today the stem cell and regenerative medicine industries are interlinked and over the last number of years have grown substantially. Regenerative medicine replaces or regenerates cells, tissues or organs and in order to achieve this uses produces from the pharmaceutical, biologics, medical device and cell therapy spaces. Therefore, cell therapy, and stem cells come under the umbrella market of regenerative medicine. Cell therapy is a platform by which regenerative medicine can achieve its aim and concentrates on using cells as therapeutics to treat disease. In 2019, the global stem cell market was worth $x billion globally, and this is set to rise to $x billion by 2025 with a CAGR of x%.
Tissue Engineering Market Analysis and Forecast to 2025
Tissue engineering was the forerunner of the present regenerative medicine market. The area of biomaterials was developed to use cells and biological material and incorporate them into scaffolds and functional tissues. Some of the main applications of tissue engineered products include artificial skin and cartilage and so this area dominates the dermatology, bone and joint submarket. Wound repair is also a significant area for tissue engineering, with products such as Dermagraft in the market.
The global market for tissue engineering was estimated at $x billion in 2019 by Kelly Scientific analysis. It is forecast to grow at a CAGR of x% to 2025 and potentially reach $x billion. Tissue engineering is being driven by the increase in technology of biomaterials, bioscaffolds and bio 3D printing. The rise in the amount of orthopedic transplantations is demanding the market to produce more innovative solutions such as 3D printed organs. In the long term future, Kelly Scientific forecasts the advance of cutting edge 3D bioprinters in this market place.
Direct Purchase of this Global Regenerative Medicine Market Research Report at https://www.reportsnreports.com/purchase.aspx?name=4705521
Biobanking Market Analysis
The biobanking industry is made up of over 500 public and private blood banks globally. These companies and institutions collect, store and distribute adipose tissue, cord blood and birth tissues, musculoskeletal tissues, pericardium, skin, bone, vascular tissue, autologous and allogeneic cells and other biological samples. They operate by charging a collection fee and then a storage fee, which is usually operational for 20 years. Private banking costs between $1,350 to $2,300 as an initial fee, and then between $100 to $175 per annum for storage. Public banking is free, and a number of hybrid models have been introduced in Europe and Asia to date.
CAR-T Industry
The CAR-T industry is addressing unmet needs in specific relapsed cancers, however does early clinical trial data support a blockbuster status for this upcoming therapy? Some patients do indeed show long term activity and high remission rates, but there is a large proportion of patients with toxicities such as cytokine release syndrome and neurotoxicity. The main players within the CAR-T market are Juno Therapeutics, Kite Pharma, Novartis and Cellectis. The market is moving ahead, backed by years of R&D, from both academia and industry, investors capitol and small clinical studies. From 2020, Kelly Scientific forecasts that CAR T therapy will become more streamlined, with faster manufacturing times as advances in technologies take hold and clinical trials provide more robust evidence that this immunotherapy is robust. These factors, plus strategies to reduce adverse reactions and toxicities and larger players like Novartis taking stage will push CAR T therapy ahead. However, recent deaths in the Juno ROCKET trial are creating questions amongst investors. How will the CAR T space influence the total immunotherapy industry going forward? This comprehensive report scrutinizes the total market and provides cutting-edge insights and analysis.
Key Questions Answered
• What is the global market for regenerative medicine from to 2025?
• What is the global market for regenerative medicine by geography, through 2025?
• What is the global market for regenerative medicine by indication, through 2025?
• What is the global market for the stem cell industry to 2025?
• What is the global market for the stem cell industry by geography, through 2025?
• What is the global market for the stem cell industry by indication, through 2025?
• What disruptive technology is advancing the overall regenerative medicine market?
• What are the major company players in the regenerative medicine, stem cells, tissue engineering and CAR-T industries?
• What types of clinical trials are currently being performed by stakeholders and major players?
• What are the strengths, weaknesses, opportunities and threats to the market?
• Which geographic markets are dominating the space?
• What are the advantages and disadvantages of the allogenic and autologous stem cell industry?
• What are the main driving forces of this space?
• What are the main restraints of the regenerative medicine industry as a whole?
• What is the current environment of the global cord blood bank industry?
• What are the global access challenges of the regenerative medicine market?
• What is the space like in Japan, China, South Korea, USA and Europe?
• What are the main challenges associated with CAR T therapy?
• When will the first CAR T therapeutics be approved?
• What are the current regulations for immunotherapies in USA, Europe & Japan?
• What are the main manufacturing steps in CAR T therapy?
• What challenges lie ahead for CAR T production?
Table of Contents:
1.0 Report Synopsis
1.1 Objectives of Report
1.2 Executive Summary
1.2 Key Questions Answered in this Report
1.3 Data Sources and Methodology
2.0 Introduction
2.1 Gurdon and Yamanaka Share the Nobel Prize
2.2 Stem Cell Clinical Trials: Initiated in 2010
2.3 Types of Stem Cells
2.4 Adult (Tissue) Stem Cells
2.5 Pluripotent Stem Cells
2.6 Somatic Cell Nuclear Transfer (SCNT)
2.7 Induced pluripotent Stem Cells (iPSC)
2.8 Mesenchymal Cells
2.8.1 MSCs in the Bone Marrow Stroma
2.8.2 Isolation, Marker Specificity and Functional Properties of MSCs
2.8.3 Oxygen Concentration and MSC Culture
2.8.4 Assays to Define MSCs
2.8.5 Differentiation Potential of MSCs
2.8.6 Therapeutic Potential of MSCs
2.8.7 Mesenchymal Stem Cells & Chronic Disease
2.8.8 MSCs and Amylotrophic Lateral Sclerosis
2.8.9 MSCs and Parkinson’s Disease
2.8.10 MSCs and Alzheimer Disease
2.8.11 MSCs and Rheumatoid Arthritis
2.8.12 MSCs and Type 1 Diabetes
2.8.13 MSCs and Cardiovascular Disease
2.9 Hematopoietic Stem and Progenitor Cells
2.9.1 In Vivo Assays for Hematopoietic Stem Cells
2.9.2 In Vitro Assays for Hematopoietic Stem and Progenitor Cells
2.9.3 Isolation of Hematopoietic Stem and Progenitor Cells
2.9.4 Culture of Hematopoietic Cells
2.9.5 Therapeutic uses of Hematopoietic Cells
2.10 Umbilical Cord Stem Cells
2.11 Heart Stem Cells
2.11.1 Cutting Edge Research in Heart Stem Cells
2.12 Mammary Stem Cells
2.12.1 Defining the Mammary Stem Cell
2.12.2 Influence of Model System on Stem Cell Identification
2.12.3 Breast Cancer Stem Cells
2.13 Neural Stem Cells
2.13.1 Identification of Neural Stem Cells
2.13.2 Function of Neural Stem Cells in Vivo
2.13.3 Neural Stem Cell Culture Systems
2.13.4 Isolation Strategies for Neural Stem Cells
2.13.5 Brain Tumour Stem Cells
2.13.6 Cutting Edge Research in Neural Stem Cells
2.14 Stem Cell Applications in Retinal Repair
2.14.1 Embryonic Stem Cells as Retina Therapeutics
2.14.2 Induced Pluripotent Stem Cells as Retina Therapeutics
2.14.3 Adipose Derived Mesenchymal Stem Cells as Retina Therapeutics
2.15 Liver Stem Cells
2.16 Gut Stem Cells
2.16 Pancreatic Stem Cells
2.17 Epidermal Stem Cells
3.0 Stem Cells and Clinical Trials
3.1 Introduction
3.2 Pluripotent Stem Cells
3.3 Limbal Stem Cells
3.4 Neural Stem Cells
3.5 Endothelial Stem or Progenitor Cells
3.6 Placental Stem Cells
3.7 Why Do Stem Cell Clinical Trials Fail?
3.8 What is the Future of Stem Cell Trials?
3.9 Cutting Edge Stem Cell Clinical Trials
3.10 Ocata Therapeutics Current Stem Cell Trials
3.11 CHA Biotech Current Stem Cell Trials
3.12 Pfizer Current Stem Cell Trials
3.13 GSK Current Stem Cell Trials
3.14 Bayer Current Stem Cell Trials
3.15 Mesoblast International Current Stem Cell Trials
3.16 Millennium Pharmaceutical Current Stem Cell Trial
3.17 AstraZeneca Current Stem Cell Trials
3.18 Merck Current Stem Cell Trials
3.19 Chimerix Current Stem Cell Trials
3.20 Eisai Current Stem Cell Trials
3.21 SanBio Current Stem Cell Trials
3.22 Celgene Current Stem Cell Trials
3.23 StemCells Current Stem Cell Trials
3.24 Genzyme (Sanofi) Current Stem Cell Trials
3.25 Teva Current Stem Cell Trials
3.26 MedImmune Current Stem Cell Trials
3.27 Janssen Current Stem Cell Trials
3.28 Seattle Genetics Current Stem Cell Trials
3.29 Baxter Healthcare Current Stem Cell Trials
3.30 InCyte Corp Current Stem Cell Trials
4.0 Stem Cells, Disruptive Technology, Drug Discovery & Toxicity Testing
4.1 Introduction
4.2 Case Study: Genentech and Stem Cell Technology
4.3 3D Sphere Culture Systems
4.4 Stem Cells and High Throughput Screening
4.4.1 Embryonic Stem Cells
4.4.2 Adult Stem Cells
4.4.3 Opportunities & Challenges
4.5 Genetic Instability of Stem Cells
4.6 Comprehensive in Vitro Proarrhythmia Assay (CiPA) & Cardiomyocytes
4.8 Coupling Precise Genome Editing (PGE) and iPSCs
4.9 Stem Cells & Toxicity Testing
4.9.1 Hepatotoxicity and iPSCs
4.9.2 Cardiotoxicity and iPSCs
4.9.3 Neurotoxicity and iPSCs
4.10 Stem Cell Disease Models
4.11 Defining Human Disease Specific Phenotypes
4.11.1 Molecular Phenotypes for Disease Modelling
4.11.2 Cellular Phenotypes for Disease Modelling
4.11.3 Physiological Phenotypes for Disease Modelling
4.11.4 Parkinson’s Disease
4.11.5 Alzheimer’s Disease
4.11.6 Amyotrophic Lateral Sclerosis
4.11.7 Huntington’s Disease
4.11.8 Spinal Muscular Atrophy
4.11.9 Down Syndrome
4.11.10 Cystic Fibrosis
4.11.11 Colorectal Cancer
4.12 Advantages of Stem Cell Derived Cells & Tissues for Drug Screening
5.0 Stem Cell Biomarkers
5.1 Pluripotent Stem Cell Biomarkers
5.2 Mesenchymal Stem Cell Biomarkers
5.3 Neural Stem Cell Biomarkers
5.4 Hematopoietic Stem Cell Biomarkers
6.0 Manufacturing Stem Cell Products
6.1 Manufacturing Strategies For Stem Cell Products
6.2 BioProcess Economics for Stem Cell Products
6.3 Capital Investment
6.4 Cost of Goods
6.5 Bioprocess Economic Drivers & Strategies
6.6 hPSC Expansion & Differentiation using Planar Technology
6.7 hPSC Expansion using 3D Culture
6.8 Microcarrier Systems
6.9 Aggregate Suspension
6.10 Bioreactor Based Differentiation Strategy
6.11 Integrated hPSC Bioprocess Strategy
6.12 GMP Regulations and Stem Cell Products
7.0 Investment & Funding
7.1 What do Investors Want from Cell & Gene Therapy Companies?
7.2 What Makes a Good Investment?
7.3 What Types of Companies do Not Get Investment?
7.4 Global Funding
7.5 Cell & Gene Therapy Investment Going Forward
7.6 What Cell & Gene Companies are the Most Promising in 2018?
7.7 Insights into Investing in Cell and Gene Therapy Companies
8.0 Regenerative Medicine Market Analysis & Forecast to 2025
8.1 Market Overview
8.2 Global Frequency Analysis
8.3 Economics of Regenerative Medicine
8.4 Market Applications & Opportunities for Regenerative Therapies
8.4.1 Neurological Disease
8.4.2 Autoimmune Disorders
8.4.3 Cardiovascular Disease
8.4.4 Diabetes
8.4.5 Musculoskeletal Disorders
8.4.6 Ocular Disease
8.4.7 Orthopedic Disorders
8.4.8 Wound Healing
8.5 Global Financial Landscape
8.6 Regenerative Medicine Clinical Trial Statistics
8.7 Regenerative Medicine Market Forecast to 2025
8.8 Regenerative Medicine Geographic Analysis and Forecast to 2025
8.9 Regenerative Medicine Geographical Location of Companies
8.10 Regenerative Medicine Technology Breakdown of Companies
8.11 Commercially Available Regenerative Medicine Products
8.12 Major Regenerative Medicine Milestones
9.0 Stem Cell Market Analysis & Forecast to 2025
9.1 Autologous & Allogenic Cell Market Analysis
9.2 Stem Cell Market by Geography
9.2.1 North America
9.2.2 Asia/Pacific
9.2.3 Europe
9.3 Stem Cell Market Forecast by Therapeutic Indication
9.3.1 Orthopedic/Musculoskeletal Stem Cell SubMarket
9.3.2 Cancer Stem Cell SubMarket
9.3.3 Cardiology/Vascular Stem Cell SubMarket Analysis
9.3.4 Wound Healing Stem Cell SubMarket Analysis
9.3.5 Skin Stem Cell SubMarket Analysis
9.3.6 Ocular Stem Cell SubMarket Forecast
9.4 Stem Cell Reagent Market Trends
10.0 Tissue Engineering Tissue Engineering Market Analysis and Forecast to 2025
10.1 Geographical Analysis and Forecast to 2025
10.1.1 North America
10.1.2 Europe
10.1.3 Asia Pacific
10.2 Geographical Analysis by Company Share
10.3 Tissue Engineering Clinical Indication Analysis & Forecast to 2025
10.3.1 Orthopedic and Musculoskeletal
10.3.2 Oncology
10.3.3 Cardiology and Vascular
10.3.4 Dermatology
10.3.5 Oral and Dental
11.0 Biobanking Market Analysis
11.1 Increasing Number of Cord Blood Banks Globally
11.2 Global Biobanking Company Sector Analysis & Breakdown
11.3 Allogenic Versus Autologous Transplant Frequency
11.4 Biobanking Market Analysis & Forecast to 2025
11.5 Major Global Players
12.0 Global Access & Challenges of the Regenerative Medicine Market
12.1 Regenerative Medicine Market in the USA
12.2 Regenerative Medicine in Japan
12.2.1 Financial Investment
12.2.2 Unconventional Company Investment in Regenerative Medicine
12.3 Regenerative Medicine in China
12.4 Regenerative Medicine in South Korea
13.0 Cell and CAR T Therapy
13.1 Challenges Relating to Cell therapy and Chimeric Antigen Receptor T Cells in Immunotherapy
13.1.1 Clinical Status of CD19 CAR-T Cells To Date
13.1.2 Clinical and Regulatory Challenges for Development of CAR T Cells
13.1.3 Key Regulatory Challenges Associated with CAR-T Development
13.1.4 Summary of Select CAR-T Products by Juno, Novartis and Kite
13.1.5 Clinical Benefit Versus Toxicity in CD19-Directed ALL Clinical Trials
13.1.6 How to Manage Toxicity of CAR-T Therapy
13.2 Regulations Pertaining to Immunotherapy, including Adoptive Cell Therapy (CAR-T and TCR) Immunotherapy Regulation in the USA
13.2.1 Center for Biologics Evaluation and Research (CBER)
13.2.2 Compliance and Surveillance
13.2.3 Extra Resources on Immunotherapeutics from the FDA
13.2.4 Cellular, Tissue and Gene Therapies Advisory Committee
13.2.5 Consumer Affairs Branch (CBER) Contact in FDA
13.2.6 FDA Regulations Pertaining to Immunotherapies
13.2.7 Case Study Ovarian Cancer Immunotherapy Regulations
13.2.7.1 Efficacy
13.2.7.2 Adverse Effects
13.2.8 Trial Design Considerations for Immunotherapy
13.2.9 Development of Immune-Related Response Criteria (irRC) & Clinical Endpoints Specific to Immunotherapies
13.3 Regulations for Cell Therapy & Immunotherapy in Japan
13.3.1 PMDA and Cell Therapy & Immunotherapy
13.3.2 Increasing the Efficiency in Cell Therapy & Immunotherapy Regulatory Review
13.3.3 Forerunner Review Assignment System
13.3.4 Revised Guidelines for Clinical Evaluation of Anti‐Malignant Tumor Agents
13.3.5 Key Contacts Within the PMDA for Cell Therapy & Immunotherapeutics
13.4 European Regulation and Cell Therapy & Immunotherapeutics
13.4.1 Introduction
13.4.2 Challenges for Cell Therapy & Immunotherapy in EMEA
13.4.3 EMA Status on Potency Testing
13.4.3.1 In Vivo Potency Testing
13.4.3.2 In Vitro Potency Testing
13.4.3.3 Viable Cell Count
13.4.3.4 Autologous Cell Based Products
13.4.3.5 Reference Preparation
13.4.3.6 Adjuvant Containing Immunotherapy Products
13.4.4 EMA Status on Identifying hyper, Hypo or non-Responders
13.4.5 Challenges Relating to Biomarkers in Immunotherapy
13.4.6 Challenges Relating to Chimeric Antigen Receptor T Cells in Immunotherapy
13.4.7 Estimating Optimal Cut-Off Parameters
13.4.8 EU-Approved Immunotherapies in Melanoma
13.4.9 Key Contacts Within EMA for Cell Therapy & Immunotherapeutics
13.5 Manufacturing of Immunotherapies
13.5.1 Introduction
13.5.2 Generation of CAR-Modified T Cells
13.5.3 What Co-Stimulation and Activity Domain is Optimal to Use?
13.5.4 Optimizing Cell Culture Media
13.5.5 Manufacturing Lentiviral Vectors
13.5.6 Detection of Integrated CAR-Expressing Vectors
13.5.7 Donor Lymphocyte Infusion Procedure
13.5.8 Ex Vivo Costimulation & Expansion of Donor T Cells
13.5.9 Infusion to the Patient
13.5.10 Manufacturing Devices and Instruments Required for Immunotherapy Production
13.5.10.1 Leukapheresis
13.5.10.2 Cell Counters and Analyzer
13.5.10.3 Cell Seeding, Growth and Propagation
13.5.11 Good Manufacturing Procedure (GMP) for Immunotherapy
13.5.12 Case Study Production of Lentivirus Induced Dendritic Cells under GMP Conditions
13.5.13 Quality Control
13.5.14 Regulatory Affairs
13.5.15 Key Challenges in Manufacturing
13.5.15.1 Electroporation of T-cells
13.5.15.2 Allogenic CAR T cells
13.5.15.3 Relapse Rates are Critical
13.5.15.4 Antigen Negative Relapse
13.5.15.5 Incorporating Suicide Genes
13.5.16 Automation in Cell Therapy Manufacturing
13.5.17 Autologous Cell Therapy Manufacture Scale Up
13.6 Supply Chain & Logistics
13.6.1 Introduction
13.6.2 Case Study: Juno Therapeutics
13.7 Pricing & Cost Analysis
13.7.1 Introduction
13.7.2 CAR T Therapy Market Evaluation
13.7.3 Current Deals Within the CAR T Market
13.8 CAR-T Therapy and Solid Tumors
13.8.1 Challenges for Solid Tumors
13.8.1.1 Off-Tumor Toxic Responses
13.8.1.2 Poor Penetration to Tumor Site
13.8.1.3 Increasing Therapeutic Efficiency
13.8.2 Avoiding Immunosuppression within Tumor Microenvironment
13.8.3 Clinical Trials Show Promise
14.0 Company Profiles
14.1 Astellas Institute for Regenerative Medicine (Ocata Therapeutics)
14.1.1 Company Background
14.1.2 Products
14.1.3 Financials
14.1.4 Company Strategy
14.2 Athersys
14.2.1 Company Background
14.2.2 Products
14.2.3 Financial Analysis
14.2.4 Company Strategy
14.3 Baxter International (Baxalta, Shire)
14.3.1 Company Background
14.3.2 Financial Analysis
14.3.3 Company Strategy
14.4 Caladrius Biosciences (NeoStem)
14.4.1 Company Details
14.4.2 Products
14.4.2.1 CLBS20
14.4.2.2 CLBS03 Treg Cellular Therapy
14.4.2.3 CLBS12 CD34 Cell Therapy
14.4.3 Financial Analysis
14.4.4 Company Strategy
14.5 Cynata Therapeutics
14.5.1 Company Background
14.5.2 Product Details
14.5.3 Financial Data
14.5.4 Company Strategy
14.6 Cytori Therapeutics
14.6.1 Company Products
14.6.2 Financial Analysis
14.6.3 Company Strategy
14.7 MEDIPOST
14.7.1 Company Details
14.7.2 Company Products
14.7.2.1 CellTree Umbilical Cord Blood Bank
14.7.2.2 CARTISTEM®
14.7.2.3 NEUROSTEM®
14.7.2.4 PNEUMOSTEM ®
14.7.3 Financial Analysis
14.8 Mesoblast
14.8.1 Company Details
14.8.1.1 Unique Features of Mesoblast and its Disruptive Technology
14.8.1.2 Allogeneic Mesenchymal Lineage Adult Stem Cells (MLCs)
14.8.1.3 Mechanism of Action of MLC Products
14.8.1.4 Manufacturing of Mesoblast MLC-Based Products
14.8.1.5 Mesoblast Patent Portfolio
14.8.2 Mesoblast Product Portfolio
14.8.2.1 MSC-100-IV/TEMCELL® for Acute Graft Versus Host Disease (aGVHD)
14.8.2.2 MPC-150-IM - Chronic Heart Failure (CHF)
14.8.2.3 MPC-25-IC for Acute Myocardial Infarction
14.8.2.4 MPC-06-ID - Chronic Low Back Pain (CLBP) due to Degenerative Disc Disease (DDD)
14.8.2.5 MPC-300-IV for Biologic-Refractory Rheumatoid Arthritis
14.8.2.6 MPC-300-IV for Diabetic Nephropathy
14.8.2.7 MPC-100-IV for Crohn’s Disease
14.8.2.8 MPC-25-Osteo for Spinal Fusion
14.8.3 Mesoblast International Strategic Business Collaborations
14.8.4 Mesoblast Financial Analysis
14.9 NuVasive
14.9.1 Company Details
14.9.2 Biologic Products for the Spinal Surgery Market
14.9.2.1 Formagraft
14.9.2.2 AttraX
14.9.2.3 Propel DBM
14.9.2.4 Osteocel Plus and Pro
14.9.3 Financial Analysis
14.9.4 Company Business Strategy
14.10 Osiris Therapeutics
14.10.1 Company Profile
14.10.1.1 BioSmart Cryopreservation Technology
14.10.1.2 MSC Primer Technology
14.10.2 Products
14.10.2.1 Grafix
14.10.2.2 BIO 4
14.10.2.3 Cartiform
14.10.2.4 Stravix
14.10.3 Company Financial Analysis
14.10.4 Company Strategy
14.11 Plasticell
14.11.1 Company Profile
14.12 Pluristem Therapeutics
14.12.1 Company Profile
14.12.2 Products
14.12.2.1 PLacental eXpanded (PLX) Cells
14.12.2.2 PLX-PAD
14.12.2.3 PLX-R18
14.12.3 Financial Analysis
14.12.4 Business Strategy
14.13 Pfizer
14.13.1 Company Profile
14.14 StemCells Inc
14.14.1 Company Profile
14.14.2 HuCNS-SC Platform Technology
14.14.3 Clinical Trial Analysis
14.14.4 Financial Analysis
14.15 STEMCELL Technologies
14.15.1 Company Details
14.15.2 Product Details
14.16 Takara Bio
14.16.1 Company Details
14.16.2 Product Portfolio
14.16.2.1 HF10 Anti-Cancer Therapy
14.16.2.2 TCR Gene Therapy
14.16.2.3 MazF Gene Therapy
14.16.3 Centre for Cell and Gene Processing
14.16.4 Company Financials
14.16.5 Company Strategy
14.17 Tigenix
14.17.1 Company Background
14.17.2 Products
14.17.3 Financial Data
14.17.4 Company Strategy
15.0 SWOT Industry Analysis
15.1 What has Strengthened the Industry Thus Far?
15.2 Allogenic and Autologous Stem Cell Industry SWOT Analysis
15.3 What are the Main Driving Forces of this Space?
15.4 Restraints of the Regenerative Medicine Industry as a Whole
15.5 Industry Opportunities Within this Sector
15.6 USA SWOT Analysis
15.6.1 Growth Opportunities
15.6.2 Drivers
15.6.3 Market Challenges
15.7 UK SWOT Analysis
15.7.1 Growth Opportunities
15.7.2 Drivers
15.7.3 Market Challenges
15.8 South Korea SWOT Analysis
15.8.1 Growth Opportunities
15.8.2 Drivers
15.8.3 Market Challenges
15.9 China SWOT Analysis
15.9.1 Growth Opportunities
15.9.2 Drivers
15.9.3 Challenges
15.10 Japan SWOT Analysis
15.10.1 Opportunities
15.10.2 Market Drivers
15.10.3 Challenges
15.11 Singapore SWOT Analysis
15.11.1 Opportunities
15.11.2 Market Drivers
15.11.3 Challenges
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
+ 1 888 391 5441
sales@reportsandreports.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Regenerative Medicine Market Key Players Analysis Ocata Therapeutics, Athersys, Baxter International, Caladrius Biosciences, Cynata Therapeutics, Cytori Therapeutics, MEDIPOST, Mesoblast here
News-ID: 2341381 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
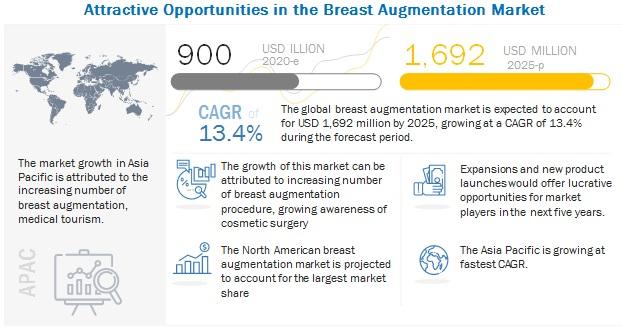
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…