Press release
SARS-CoV-2 COVID Vaccine Market Analysis by Company, Leading Players, Geography and Segments | Vaccine Technology – mRNA, Recombinant Protein, Viral Vectors, whole inactivated virus
This report provides a comprehensive overview of the size of the SARS-CoV-2 COVID Vaccine market, segmentation of the industry (by geography and vaccine technology), key players and the vast potential of vaccines that are in clinical trials. Kelly Scientific analysis indicates that the global COVID vaccine market was worth $59 billion in 2021. However, our forecast indicates that an initial drop in 2022 will occur due to a number of factors including single booster shots as opposed to two shot vaccines and reduced prices in vaccines due to competition.Get a Free Sample Copy of this Global SARS-CoV-2 COVID Vaccine Market Research Report at https://www.reportsnreports.com/contacts/requestsample.aspx?name=4186838
The COVID vaccine industry is expected to then grow significantly at a CAGR of over 9% and reach $47.5 billion by 2026. This is due to an opening up of the market from a closed government dominated space to one in which private healthcare providers and companies can purchase vaccines. There will also be a continued significant un-met need for vaccine boosters in an endemic situation.
Countries Covered
North America, Europe, Japan, UK, RoW
This report describes the evolution of such a huge market in 8 chapters supported by over 74 tables and figures in over 130 pages. The report focuses on the following:
• An overview of the SARS-CoV2 virus that includes: genetic and structural analysis, mutation and variant characterization
• Global COVID vaccine market, global landscape analysis, vaccine technology breakdown and leading market players
• Advance purchase agreements, marketed/pipeline products, financial analysis and business strategy of the major companies in this space
• Focus on current trends, business environment, pipeline products, clinical trials, and future market forecast for COVID vaccines to 2026
• Clinical trial timelines for major vaccines in the pipeline
• Insight into the challenges faced by stakeholders, particularly manufacturers and governments
• Insight into the emergence of variants and their impact on vaccine effectiveness and the overall market
• Financial market forecast through 2026 with CAGR values of all market segments – vaccine technology, geography (US, EU, UK, Japan, RoW) and major players
• Influence of the molecular diagnostics, PCR testing and POC testing landscapes
• Numbers of vaccine doses available by each manufacturer per annum
• Geographical analysis and challenges within countries with respect to advance purchase agreements and distribution channels
Get 25% Discount on Global SARS-CoV-2 COVID Vaccine Market Research Report at https://www.reportsnreports.com/contacts/discount.aspx?name=4186838
Global SARS-CoV-2 Outbreak – Pandemic to Vaccine Discovery
Since an initial outbreak of SARS-CoV-2 in Wuhan at the end of 2019, to a pandemic of over 110 million infections and 2.41 million deaths in 2021, a global effort has been made to structurally elucidate this virus and develop new vaccines to reduce its transmission. Currently, Pfizer/BioNTech, Moderna, AstraZeneca, Gamelaya, J&J, Novavax, Sinovac and Curevac have developed the most prominent vaccines in the pipeline. At the time of writing, 234 million people have been vaccinated globally, the majority being in the US, China and the EU.
Challenges of Mutations and Threats of Future Variants
The emergence of SARS-CoV-2 spike protein mutations globally have enabled the virus to become more infectious. Sequencing thousands of samples have enabled the identification of the most common mutations thus far including B.1.1.7 (UK), B.1.351 (South Africa) and P1 lineage (Japan/Brazil). Mutations such as these give the virus an evolutionary advantage for selection as they allow for protein structural changes in the spike protein.
This is most notable in Ireland which had a 5% prevalence of the B.1.1.7 strain in December 2020, which increased dramatically to 90% prevalence by February 2021. The B.1.1.7 strain contains numerous mutations in the spike region which give it a higher affinity to attach to human cells. Studies to date estimate that this variant is between 30-50% more infectious than the original strain.
Will this Have an Effect on the Efficacy of Vaccines?
Mutations arising in the spike protein of variants pose a potential problem for the efficacy of vaccines. This is due to the fact that vaccines to date target the spike protein, and so neutralizing antibodies and the T cell response are primed against the original spike protein structure. With regards the South African B1.351 variant, serum from individuals who received the Moderna mRNA-1273 vaccine were shown to have lower neutralization ability in in vitro assays. However, even though neutralization ability was lower, it was still significant.
A similar study demonstrated that neutralization of a virus containing three mutations from the South African variant from sera from individuals vaccinated with Pfizer/BioNTech BNT162b2 was lower than the UK variant. However, the study indicated that differences in neutralization was very small. This study indicates that the Pfizer/BioNtech vaccine still works against the South African strain, but is slightly less effective. Similarly, the AstraZeneca, Novavax and J&J vaccines also show slightly less protection.
A recent report in the New England Journal of Medicine evaluated a study group of 596,618 vaccinated people (matched to unvaccinated controls) in Israel for BNT162b2 COVID vaccine effectiveness. Their results indicated that BNT162b2 vaccination is highly effective in a real world population, and mirrors clinical trial results thus far.
Direct Purchase of Global SARS-CoV-2 COVID Vaccine Market Research Report at https://www.reportsnreports.com/purchase.aspx?name=4186838
Vaccines Penetrating the Market
Currently, there are a range of vaccines penetrating the COVID market, most notably using mRNA technology (Pfizer/BioNTech/Moderna/CureVac), recombinant proteins (Novavax), viral vectors (AstraZeneca/J&J/Gamaleya) and inactivated virus (Sinovac/Sinopharm/Bharat Biotech).
SARS-CoV-2 will continue to exist as influenza and RSV does, and so it will become an endemic virus in the future. It is forecast that we will always potentially be fighting a variant of the original virus and vaccines will adapt to this over time. Regulatory pathways will also need to adapt in concert.
Data Sources and Methodology
Based in Locations in Europe, our analyst team are all PhD-level experts and industry-experienced professionals. They pool resources, contacts, business acumen and technical experience to provide cutting edge insights for all of Kelly Scientific reports. Our senior analysts have at least ten years’ experience in major strategic corporations. Our methodologies are clearly defined from the outset. Initially a number of clear objectives are set, e.g., to identify the market size, segmentation, key players, SWOT analysis, influential technologies, and business and economic environments:
• By Company (e.g., Pfizer. BioNTech, Moderna, AstraZeneca, Novovax, J&J)
By Geography (US, UK, EU, Japan, RoW)
• By Segment (vaccine technology – mRNA, Recombinant Protein, Viral Vectors, whole inactivated virus)
Key Strengths, Weaknesses and Threats Influencing Leading Player Position within the Market:
• Technologies Driving the Market
• Top Fastest Growing Market Segments and Emerging Opportunities
• Top Pharmaceutical Companies within the by Market Share and Revenue
• Comprehensive Product Portfolios, R&D Activity and Pipeline Therapeutics
• M&A Activity and Future Strategies of Top Pharmacos
Following this, we determine key financial data & business strategy information to give clients the most accurate information required to identify areas of profitable growth and what technical advantages are required in a competitive landscape:
• Company Financials, Sales & Revenue Figures
• Government advance purchase agreements (ADA’s) and funding
• Business Model Strategies for Diagnostic, Pharmaceutical and Biotechnology Companies
Market analysis is initiated using primary research tools such as speaking directly with end-users, identifying their needs and any un-met needs in the market place. This exploratory research identifies any specific requirements in the market, and is tailored specifically to niche markets such as COVID vaccines, immunotherapy, personalized medicine, targeted therapeutics and companion diagnostics, drug delivery systems, cosmetic surgery and services, and cancer biomarkers. At Kelly Scientific, we have a wide range of contacts within these niche areas that provide us with cutting edge insights to a marketplace that is beyond the reach of many. We also travel to a wide range of international conferences quarterly to source new data and trends from global experts.
Secondary research performed by Kelly Scientific is meticulously scrutinized and analysed prior to integration into a final report. We only use validated and confirmed sources of information from company specific corporate websites, annual reports, press-releases, government publications, international scientific and medical journals and research reports. All graphical and numerical data included in our reports are referenced and sourced accordingly. Specific websites are consulted and referenced throughout the including that of the Food and Drug Association (www.fda.gov), the National Cancer Institute, World Health Organization, PubMed, Clinicaltrials.gov and other government agencies worldwide. Kelly Scientific utilises the most recent statistical and numerical data available.
Details at https://www.prnewswire.com/in/news-releases/sars-cov-2-covid-vaccine-market-demands-growth-size-supply-and-key-players-are-pfizer-biontech-moderna-astrazeneca-novovax-j-amp-j-and-others-reportsnreports-827927654.html
Together both primary and secondary research and also unique insights from the chief analysts and editor alike provide the client with a report that exceeds its competitors. We strive to out-perform our competitors, just like our clients.
The senior editor of the SARS-CoV-2 COVID Vaccine market analysis report obtained a Ph.D. in Medicine/Genetics/Immunology from the Royal College of Surgeons in Ireland, a M.Sc. in Biotechnology (NUIG) and an honours degree in Biochemistry from Trinity College Dublin. With many years of medical writing, publishing, market analysis and forecasting, the senior editor also has extensive knowledge of immunology, vaccine development, infectious diseases, diagnostic testing and molecular biology.
Table of Contents:
1.0 Introduction
1.1 Emergence of SARS-CoV2 and the COVID Pandemic
1.2 SARS-CoV-2 Protein and RNA Structure
1.3 SARS-CoV-2 Infection and Immune Response
1.4 SARS-CoV-2 Mutations
1.4.1 B.1.1.7 Variant
1.4.2 B.1.351 Variant
1.4.3 P.1 Variant
1.4.4 Rapid Spread of D614G Mutation Globally
1.4.5 Variants and Vaccine Efficacy
1.4.6 BNT162b2 mRNA Covid-19 Vaccination Effectiveness in Israel
1.4.7 Challenges of Mid-Term and Long-Term Mutations
1.5 Future of Living Alongside SARS-CoV-2
2.0 COVID Vaccines
2.1 Introduction 2.1.1 mRNA Vaccines
2.1.2 DNA Vaccines
2.1.3 Inactivated Vaccines
2.1.4 Live-Attenuated Vaccines
2.1.5 Recombinant Protein Vaccines
2.1.6 Viral Vector Vaccines
2.2 Pfizer/BioNTech BNT162b2 Vaccine
2.3 Moderna mRNA1273 Vaccine
2.4 CureVac CVnCoV Vaccine
2.5 AstraZeneca/Oxford AZD-1222 Vaccine
2.6 Johnson & Johnson’s JNJ-78436735 Vaccine
2.7 Novavax NVX-CoV2373 Vaccine
2.8 Gamaleya Sputnik V Vaccine
2.9 Sinovac Coronavac Vaccine
3.0 Clinical Trials
3.1 Introduction
3.2 Clinical Trials by Geographic Area
3.3 Clinical Trials by Phase
3.3.1 Phase III COVID Vaccine Trials
3.3.2 Phase II COVID Vaccine Trials
3.3.3 Phase I COVID Vaccine Trials
4.0 Molecular Diagnostics & PCR Testing
4.1 Introduction
4.2 PCR Diagnostic Testing Has Dominated the Space
4.3 Point of Care Testing
5.0 Market & Industry Analysis
5.1 Advance Purchase Agreements by Company
5.1.1 Pfizer/BioNTech
5.1.2 Moderna
5.1.3 AstraZeneca/Oxford
5.1.4 Novavax
5.1.5 Sanofi/GSK
5.1.6 J&J
5.1.7 Curevac
5.2 Advance Purchase Agreements - Geographical Breakdown
5.2.1 USA
5.2.2 EU
5.2.2.1 Distribution of Vaccines within the EU
5.2.3 Japan
5.2.4 Australia
5.2.5 China
5.2.6 UK
5.2.7 Russia
5.3 Price of Vaccines
5.4 Reasons for Booster Vaccine Requirements
6.0 COVID Vaccine Pandemic Market Analysis 2021
6.1 Market Revenue, Volume and Major Players
6.2 SubMarket by Major Player Breakdown
6.3 SubMarket by Technology Type
6.4 SubMarket by Geographical Breakdown
7.0 COVID Vaccine Market 2022 to 2026
7.1 Endemic Market Forecast to 2026
7.2 SubMarket by Geographical Breakdown to 2026
7.3 SubMarket by Major Player Breakdown to 2026
7.4 SubMarket by Vaccine Technology Breakdown to 2026
8.0 Future Outlook
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
+ 1 888 391 5441
sales@reportsandreports.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release SARS-CoV-2 COVID Vaccine Market Analysis by Company, Leading Players, Geography and Segments | Vaccine Technology – mRNA, Recombinant Protein, Viral Vectors, whole inactivated virus here
News-ID: 2340642 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
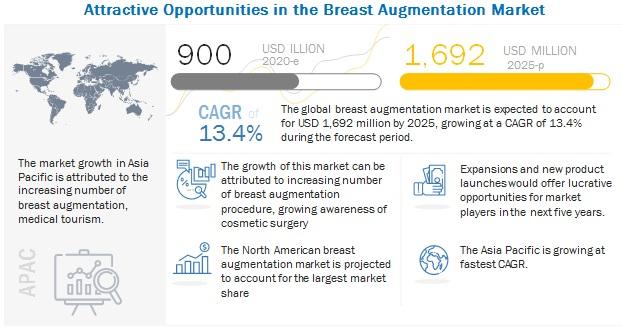
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Vaccine
Adult Vaccines Market By Type (Preventive Vaccine, Therapeutic Vaccine)
Global Adult Vaccines Market 2022 Research Report initially provides a basic overview of the industry that covers definition, applications and manufacturing technology, post which the report explores into the international players in the market.
Download Sample PDF at https://www.theinsightpartners.com/sample/TIPRE00007244/?utm_source=OpenPR&utm_medium=10379
Key Players Analysis:
AstraZeneca
Bharat Biotech
Dynavax Technologies Corporation
GlaxoSmithKline
Johnson & Johnson
Merck and Co
Novartis
Pfizer
Sanofi Pasteur
Serum Institute of India
The report covers key developments in the Adult Vaccines market as organic and inorganic growth strategies. Various companies are focusing…
Flu Vaccine (Influenza Vaccine) Market: A look into the future of the “Univers …
Latest update on Flu Vaccine (Influenza Vaccine) Market Analysis Report published with an SMI, the industry growth analysis, and Projection by 2021-2028. This report is highly predictive as it holds the overall market analysis of the topmost companies in the Flu Vaccine (Influenza Vaccine) industry. With the classified Flu Vaccine (Influenza Vaccine) market research based on various growing regions, this report provides leading players' portfolios along with sales, growth, market…
Vaccine Market
Rise in the prevalence of infectious diseases, increase in immunization programs across the globe, and surge in R&D activities to develop new vaccine drive the growth of the global vaccine market.
Global Vaccines Market was pegged at $32.46 billion in 2019, and is anticipated to hit $54.15 billion by 2027, registering a CAGR of 6.6% from 2020 to 2027. The report provides an in-depth analysis of the top investment pockets, top…
US Flu Vaccine [Influenza Vaccine] Market Research Report 2020
DPI Research offers a latest published report on “Influenza Vaccines in the United States – Market Size, Trends, Opportunities and Growth Potential” delivering key insights and providing a competitive advantage to clients through a detailed report. This report focuses on the key US flu vaccines market players, to define, describe and analyze the value, market share, market competition landscape, SWOT analysis and development plans in next few years. To analyze…
Measles Vaccine
Measles Vaccine Market describes its growth, size, share, Forecast and trends to 2025
Measles Vaccine Market Production and Demand Analysis 2019 to 2025
Measles Vaccine Market 2019 Manufacturing Analysis and Development Forecast 2025
Measles Vaccine Market 2019: Recent Study Including Growth Factors, Regional Drivers, Forecast 2025
Measles Vaccine Market to Insight By 2025: Top Key Vendors
The global Measles Vaccine market is valued at million US$ in 2018 and will reach million US$ by the…
Monovalent vaccine segment to accumulate maximum Paediatric Vaccine Market share
According to a new report published by Future Market Insights titled “Paediatric Vaccine Market: Global Industry Analysis & Opportunity Assessment, 2016 – 2026”, in terms of revenue, the global paediatric vaccine market is expected to increase at 12.2% CAGR during the forecast period 2016-2026. The global paediatric vaccine market is expected to reach US$ 27.97 Bn in 2016.
Paediatric vaccine market is a billion dollar market accounting for a substantial proportion…