Press release
Clinical Trial Market by Phases, Region, Companies, Global Forecast by 2026
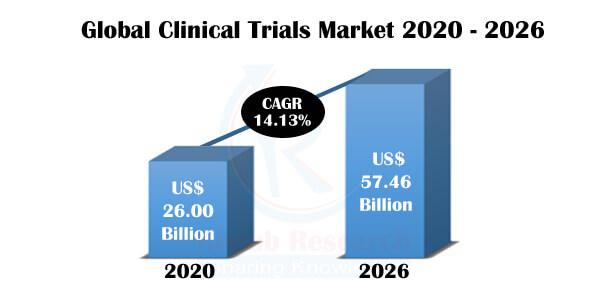
It expected that the Global Clinical Trials Market will expand, registering a CAGR of 14.13% during the forecast period, 2020-2026
After preclinical development, the investigational new drug passes through clinical phases I, II, III and IV during the clinical trial. These phases explain pharmacokinetics, pharmaco dynamics profile and side effect, which may be harmful or beneficial, adverse impact and post-marketing surveillance. Furthermore, as per our analysis, the market is anticipated to be dominated by Phase III, with Phase I expected to behold the fastest growth.
Factors Driving the Clinical Trials Industry Worldwide
The significant factors propelling the market of the global clinical trials market are increasing new medical equipment demand and medicines among end-users, coupled with growing investment for research and development activities for the development of effective medications. Moreover, the increasing number of individuals suffering from chronic diseases and evolving circumstances & nature of certain types of chronic diseases are other factors anticipated to support the growth of the global clinical trials market to a significant extent. It expected that the Global Clinical Trials Market will expand, registering a CAGR of 14.13% during the forecast period, 2020-2026.
Request a Free Sample Copy of the Report: https://www.renub.com/request-sample-page.php?gturl=clinical-trials-market-p.php
By Indication
The Oncology segment held the most considerable market presence in the clinical trials market in 2020. Clinical trials are acting as the key to making progress against cancer. Today, people live longer lives from successful cancer treatments that are the results of past clinical trials. Clinical trials have helped to find new ways to prevent and detect cancer. And have also improved the quality of life for people during and after treatment. According to Renub Research, the Clinical Trials Market is valued at US$ 26.00 Billion in 2020.
By Study Designs - Global Clinical Trial Industry
The Interventional Design segment has the most prominent presence in the market. In addition, the Interventional Design in oncology is a rapidly growing sub-speciality that aims to develop new disease-modifying treatment options beyond conventional surgical and oncological therapies in several disease settings. The evidence for interventional oncology success dominated by single-arm study reporting technical success or clinical efficacy.
Clinical Trial Regions
North America is a conventional clinical trial region, and because of the legal, regulatory considerations, the clinical trial market shifted to developing nations. In the United States, the Clinical trials are funded and sponsored by the National Institute of Health (NIH), government agencies, academic groups, voluntary health organizations and industry.
The Asia-Pacific is emerging as a natural choice and ultimate destination for the clinical trial industry. Asia-Pacific has become the most preferred destination for global clinical trials because of significant cost advantage and other resource advantages combined with the unique benefits served by Asia Pacific's 3 'P's— Population, Patients and Physicians. Numerous pharmaceutical organizations and clinical research firms have started extracting the vast potential of Asia-Pacific and conducting clinical trials in Asia-Pacific on a big scale.
The report has also analyzed the competitive landscape of the following key players: ICON Plc, Wuxi AppTec, SGS SA, Syneos Health and PRA Health Sciences Inc. Our research shows that there will be a notable increase in the efficiency and effectiveness of clinical trials in the coming years.
Disruptions Caused Due to the Coronavirus Crisis:
The COVID-19 pandemic has remarkably impacted the global market for clinical trials, as there has been a rising focus on developing new therapeutics or vaccines to curb or operate the disease. Also, COVID-19 has induced a slight shift in terms of the way clinical trials performed.
Earlier, the global clinical trial space had been experiencing an increased interest in virtual/decentralized trials. The virtual/decentralized trials have also highlighted on conference agendas and magazine articles for a long time. Finally, with the COVID-19 outbreak, the trials have been forced to move to a virtual model to keep them on track during this pandemic situation.
Renub Research latest report titled "Global Clinical Trial Market by Phases (1, 2, 3, 4), Indications (Autoimmune/Inflammation, Pain management, Oncology, CNS Condition Diabetes, Obesity ,Cardiovascular, Others), Study design (Interventional Trial Market, Observational Trial Market, Expanded Access Trial Market), Region (North America, Europe, Asia Pacific , Latin America, Middle East & Africa), Company (ICON Plc, Wuxi AppTec, SGS SA, Syneos Health, PRA Health Sciences Inc)" provides a detailed analysis of Clinical Trial Industry.
Follow the link for the full report with detailed TOC and list of figures and tables: https://www.renub.com/clinical-trials-market-p.php
Phases – Clinical Trials Market breakup from 4 viewpoints:
1. Phase 1
2. Phase 2
3. Phase 3
4. Phase 4
Indications – Clinical Trials Market breakup from 8 viewpoints:
1. Autoimmune/Inflammation
2. Pain management
3. Oncology
4. CNS Condition
5. Diabetes
6. Obesity
7. Cardiovascular
8. Others
Study Designs – Clinical Trials Market breakup from 3 viewpoints:
1. Interventional Trial Market
2. Observational Trial Market
3. Expanded Access Trial Market
Region – Clinical Trials Market breakup from 5 viewpoints:
1. North America
2. Europe
3. Asia Pacific
4. Latin America
5. Middle East & Africa
The Companies Covered have been covered from 3 viewpoints:
• Overview
• Recent Developments
• Revenue
Companies Covered:
1. ICON Plc
2. Wuxi AppTec
3. SGS SA
4. Syneos Health
5. PRA Health Sciences Inc
Industry Related Opportunity:
United States Generic Drugs Market: https://www.renub.com/united-states-generic-drugs-market-p.php
Allergic Conjunctivitis Market: https://www.renub.com/allergic-conjunctivitis-market-p.php
United States Specialty Pharmaceuticals Market: https://www.renub.com/united-states-specialty-pharmaceuticals-market-p.php
Australia Diabetes Market: https://www.renub.com/australia-diabetes-market-p.php
CAR T Cell Therapy Market: https://www.renub.com/car-t-cell-therapy-market-p.php
About the Company:
Renub Research is a Market Research and Consulting Company. We have more than 10 years of experience especially in international Business-to-Business Researches, Surveys and Consulting. We provide a wide range of business research solutions that helps companies in making better business decisions. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses. Our wide clientele comprises major players in Healthcare, Travel and Tourism, Food & Beverages, Power & Energy, Information Technology, Telecom & Internet, Chemical, Logistics & Automotive, Consumer Goods & Retail, Building, and Construction, & Agriculture. Our clients rely on our market analysis and data to make informed knowledgeable decisions. We are regarded as one of the best providers of knowledge. Our pertinent analysis helps consultants, bankers, and executives to make informed and correct decisions.
Our core team is comprised of experienced people holding graduate, postgraduate, and Ph.D. degrees in Finance, Marketing, Human Resource, Bio-Technology, Medicine, Information Technology, Environmental Science, and many more.
Contact Us:
Renub Research
Phone No: +1 678-302-0700 (USA) | +91–120–421–9822 (IND)
Email: info@renub.com
Web: https://www.renub.com
Follow on Linkedin: https://www.linkedin.com/company/renub-research
Roswell, GA 30076
Our research helps to make business decisions: on strategy, organization, operations, technology, mergers & acquisitions etc. We support many blue chip companies by providing them with findings and perspectives across a wide range of markets. Our research reports offer a blend of information insight, analysis, and forecasting that is essential in today's ultra-competitive markets.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Market by Phases, Region, Companies, Global Forecast by 2026 here
News-ID: 2320889 • Views: …
More Releases from Renub Research

White Mushroom Market to Reach US$ 77.41 Billion by 2033 at 6.39% CAGR
White Mushroom Market
White Mushroom Market is expected to reach US$ 77.41 billion by 2033 from US$ 44.33 billion in 2024, with a CAGR of 6.39% from 2025 to 2033. Rising consumer demand for nutritious, low-calorie foods, growth in ready-to-eat and processed meal categories, and expanding foodservice use drive white mushroom demand. Improved controlled-environment cultivation, year-round supply, and expanding retail distribution channels-including e-commerce-boost availability and lower prices, encouraging broader consumer adoption…

Global TAVR Market to Hit $19.69 Billion by 2033, Driven by Aging Population and …
Transcatheter Heart Valve Replacement Market Overview
The market for transcatheter heart valve replacement (THVR) is expanding significantly due to rising patient demand for minimally invasive treatments and advancements in technology. For patients with high surgical risk, transcatheter aortic valve replacement (TAVR) has emerged as the preferred option over open heart surgery. Globally, TAVR usage is growing, with North America and Europe leading the way in procedure numbers due to their sophisticated…

Global Air Conditioner Market to Reach USD 257.20 Billion by 2032, Driven by Urb …
Air Conditioner Market Analysis
Introduction
From comfort to necessity, air conditioners have become a staple in both residential and commercial spaces around the world. As global temperatures rise and urbanization accelerates, the air conditioner market continues to expand-evolving in design, technology, and energy efficiency.
This in-depth guide explores the global air conditioner market, including growth drivers, consumer trends, technological innovations, challenges, and future projections. Whether you're an investor, manufacturer, retailer, or informed buyer,…

Global Chocolate Market Analysis, Size, Share, Growth ⅼ Forecast (2024 - 2032) …
Global Chocolate Market Size was US$132.65 billion in 2023 and is expected to reach to US$196.79 billion by 2032. It is predicted to expend at a CAGR of 4.48% from 2024 to 2032.
Chocolate is a beloved treat worldwide and has been for hundreds of years. The worldwide chocolate industry is worth billions of dollars, and new trends are continuously emerging. Chocolate liquor and cocoa cream are used to make…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…