Press release
COVID-19 Impact on Plasma Therapy Market current scenario
The COVID-19 outbreak is continuing to create havoc all over the world and scientists are immensely involved to develop antidotes for the novel coronavirus, which started infecting people since late last year. Researchers and scientists are exploring several avenues to introduce the world with medical treatments that can thoroughly fight the COVID-19 disease. ‘Convalescent Plasma Therapy’ is one such treatment that is currently in focus around the globe.Presently, India has been given a go ahead for edging protocol after China and the U.S. in order to conduct a clinical trial for convalescent plasma therapy. This therapy has been already used as an experiment in the past and as a result is turning out to be a ray of hope in this war against the novel COVID-19 pandemic.
Access to FREE Sample Report Here! @ https://www.researchdive.com/download-sample/204
What is Convalescent Plasma Therapy for Coronavirus and How Does it Work?
The purpose of convalescent plasma therapy is to use antibodies from the blood of a COVID-19 recovered patient in order to treat those who are severely affected by the coronavirus. In addition, this therapy can be also used to immunize or vaccinate those at a higher risk of contracting the virus, such as family members of patients, health workers, and others more susceptible.
The concept of plasma therapy for COVID-19 is simple and based on the ground that the blood of a recovered COVID-19 patient contains antibodies with a definite ability to fight the novel coronavirus. The theory is that if the antibodies of recovered patients are ingested into the patients that are under treatment, they will start fighting and targeting the new coronavirus in the second patient. According to researchers, the convalescent plasma therapy is analogous to passive immunization and it is not a treatment but a preventive measure for the COVID-19 disease.
The antibodies from the donated blood of the recovered patient is checked for the presence of any other disease-causing agents such as HIV, Hepatitis B, Hepatitis C, etc. If reckoned safe, the blood is taken through a process of extracting ‘plasma’, which is the liquid part of the blood containing antibodies. Once extracted, the highly rich antibody plasma is then ingested into the body of a patient under treatment.
According to study authored by Liise-anne Pirofski and Casadevall, an adequate amount of antibody should be administered for effective plasma exchange therapy. When ingested into a susceptible person, this antibody will reach tissues and circulate in the blood to provide protection against the virus. Depending on the composition and antibody amount, the protection conferred by the transferred antibodies (immunoglobulin) can last from weeks to months.
Potential Risks Involved with Plasma Therapy
Though there are several benefits of plasma therapy for COVID-19, there are also a few risks involved with the therapy. Some of the potential risks associated are as follows:
Enhancement of Infection: There are slight chances that the plasma therapy might fail for a few COVID-19 patients and can result in a greater form of the infection.
Effect on Immune System: The administration of antibodies may land up suppressing the natural response of the body, leaving a COVID-19 patient susceptible to subsequent re-infection.
Transfer of Blood Substances: An inadvertent infection can possibly get transferred to the COVID-19 patient as the blood transfusion takes place while after ingesting plasma.
Speak to our Expertise before buying Plasma Therapy Market Report@ https://www.researchdive.com/connect-to-analyst/204
Past Consideration of Plasma Therapy
The convalescent plasma therapy has been considered several times as a treatment for viral infections.
A protocol for using convalescent plasma was recognized in 2015 for the treatment of MERS (Middle East Respiratory Syndrome) infection, which is also caused by a coronavirus.
The World Health Organization (WHO) in 2014, had recommended to use convalescent plasma therapy for treating patients with the antibody-rich plasma of recovered patients who were infected with the Ebola virus disease.
In 2009, plasma therapy was used to treat the H1N1 infection.
The therapy was also used experimentally during the 1918 Spanish flu or H1N1 influenza virus pandemic.
Plasma Therapy and COVID-19
The potential of plasma therapy as treatment for COVID-19 has been already explored in China in the limited trials, where the outbreak had first emerged. Around 10 highly critical COVID-19 patients were subject to plasma therapy in one trial. In this trail, a few improvements were seen in the condition of patients.
The researchers who conducted this trial stated that there were no severe adverse effects observed and that the convalescent plasma therapy for COVID-19 was well tolerated. As a result, this could potentially improve the clinical outcomes through deactivating the presence of viruses in the blood or viremia in severe COVID-19 cases.
On the other hand, the researches of Shenzhen, China also conducted another trial of five critically-ill COVID-19 patients, where in it the plasma therapy showed positive results as an improvement was seen in the patient’s conditions.
The Silver Lining
A large number of conducted studies until now have sparked a silver lining. However, it’s too early to think plasma therapy for COVID-19 as an effective treatment. According to John Hopkins University guidelines, the sample sizes in the plasma exchange therapy for Covid-19 trials are too small to for definite conclusions.
As per a published report in the Mayo Clinic’s Research magazine, number of researchers across the globe have also raised the point regarding too many unknown are still there for the treatment of COVID-19 disease. Some of the questions raised are; which patients will benefit, what should be the optimal dose of antibodies, what point does the patient should be undergone treatment, and many more. The researchers believe that these are some of the things that must be addressed before reaching actual conclusions.
The whole world today is awaiting seriously for the plasma therapy to be accepted as the potential treatment for COVID-19 disease. So while the plasma therapy for coronavirus remains a silver lining, it is expected that the world will soon get to know the treatment’s efficacy once more trials and studies are conducted.
Request for Plasma Therapy Market Report Customization & Get 10% Discount on this Report@ https://www.researchdive.com/request-for-customization/204
Contact us:
Mr. Abhishek Paliwal
Research Dive
30 Wall St. 8th Floor, New York
NY 10005 (P)
+ 91 (788) 802-9103 (India)
+1 (917) 444-1262 (US)
Toll Free: +1-888-961-4454
E-mail: support@researchdive.com
LinkedIn: https://www.linkedin.com/company/research-dive/
Twitter: https://twitter.com/ResearchDive
Facebook: https://www.facebook.com/Research-Dive-1385542314927521
Blog: https://www.researchdive.com/blog
About Us
Research Dive is a market research firm based in Pune, India. Maintaining the integrity and authenticity of the services, the firm provides the services that are solely based on its exclusive data model, compelled by the 360-degree research methodology, which guarantees comprehensive and accurate analysis. With an unprecedented access to several paid data resources, team of expert researchers, and strict work ethic, the firm offers insights that are extremely precise and reliable. Scrutinizing relevant news releases, government publications, decades of trade data, and technical & white papers, Research dive deliver the required services to its clients well within the required timeframe. Its expertise is focused on examining niche markets, targeting its major driving factors, and spotting threatening hindrances. Complementarily, it also has a seamless collaboration with the major industry aficionado that further offers its research an edge.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release COVID-19 Impact on Plasma Therapy Market current scenario here
News-ID: 2270120 • Views: …
More Releases from Research Dive

Electronic Data Management Market Expected to Rise Progressively by 2031 Due to …
The global electronic data management market is expected to witness significant growth by 2031, owing to the rising applications of electronic data management in the industrial sector. The North America region was the most dominant in 2021.
As per the report published by Research Dive, the global electronic data management market is projected to garner a revenue of $19,289.5 million and rise at a stunning CAGR of 12.2 % during…
Gastric Cancer Market Predicted to Make a Strong Comeback after the Pandemic Deb …
The global gastric cancer market is predicted to observe significant growth by 2031, owing to the increasing pervasiveness of gastric cancer among people worldwide. The Asia-Pacific region generated the highest market share in 2021.
As per the report published by Research Dive, the global gastric cancer market is envisioned to garner a revenue of $10,737.00 million and grow at a fascinating CAGR of 17.9% over the estimated timeframe from…
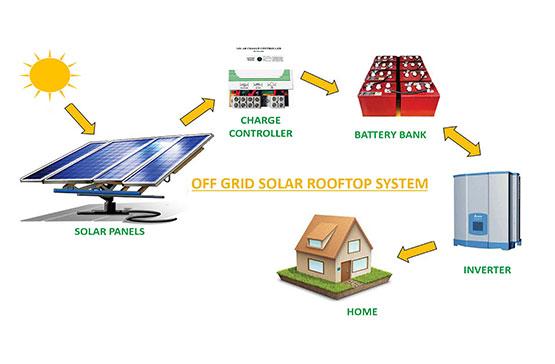
Off Grid Solar Market to Exhibit 12.3% CAGR and Generate $5,825.80 Million by 20 …
As per the report published by Research Dive, the global off grid solar market is predicted to generate a revenue of $5,825.80 million and grow at a stunning CAGR of 12.3% during the analysis timeframe from 2022 to 2031.
The global off grid solar market is predicted to witness prominent growth by 2031, owing to the increasing demand for electricity independence across the globe. The Asia-Pacific region garnered…

Roofing Materials Market to Garner a Revenue of $186.7 Billion and Exhibit a 4.3 …
As per the report published by Research Dive, the global roofing materials market is expected to register a revenue of $186.7 billion by 2031, at a CAGR of 4.3% during the forecast period 2022-2031.
The global roofing materials market is expected to grow primarily due to the growing need for waterproofing roofing materials. Re-roofing sub-segment is expected to flourish immensely. The Asia-Pacific region is predicted to grow at a high…
More Releases for Plasma
Blood Plasma Freezers Market Safeguarding Plasma Derivatives: Blood Plasma Freez …
Global Blood Plasma Freezers Market Worth $772.0 Mn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Blood Plasma Freezers Market- (By Type (Manual Defrost, Automatic Defrost), By Application (Hospital, Laboratory), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Blood Plasma Freezers Market…
Contract Plasma Coating Service Market Analysis, Size, Share, Trends, Growth And …
The Global Contract Plasma Coating Service Market report is added by WMR to its database to offer a complete assessment of the factors influencing an overall market growth trend. The research covers significant data and proves to be a handy resource document for industry experts. The research is a perfect balance bridging both qualitative and quantitative information of this market. Quantitative statistics with qualitative reasoning related to market size, share,…
Cold Plasma Market Analysis By Top Keyplayers - Plasma Air, Atmospheric plasma t …
The "Cold Plasma Market" is expected to reach USD xx.x billion by 2031, indicating a compound annual growth rate (CAGR) of xx.x percent from 2024 to 2031. The market was valued at USD xx.x billion In 2023.
Growing Demand and Growth Potential in the Global Cold Plasma Market, 2024-2031
Verified Market Research's most recent report, "Cold Plasma Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2030," provides an in-depth examination…
Contract Plasma Coating Service Market Revenue Sizing Outlook Appears Bright| He …
The Latest research study released by HTF MI "Global Contract Plasma Coating Service Market with 120+ pages of analysis on business Strategy taken up by key and emerging industry players and delivers know-how of the current market development, landscape, technologies, drivers, opportunities, market viewpoint, and status. Understanding the segments helps in identifying the importance of different factors that aid market growth. Some of the Major Companies covered in this Research…
Contract Plasma Coating Service Market Players Leveraging on Growth Opportunitie …
The Most recent study offered by "Stratagem Market Insights" focuses on Contract Plasma Coating Service Market size, share, growth rate, and market trends, as well as the parameters and factors influencing it in both the long term and short term. The report investigates the market trends in order to assess its current and future potential. Our Market analysis also provides market participants and new entrants with a comprehensive view of…
Cold Plasma Market Size, Share - Global Industry Forecast 2032 | Relyon Plasma G …
The market research report offers an in-depth analysis of the Cold Plasma market, helping players to prepare for the increasing hurdles ahead and ensure continued business expansion. With impeccable analysis, exhaustive research, and accurate forecasting, we provide a clear and authoritative study of the global Cold Plasma market, backed by data and figures that have undergone a rigorous verification process. This research is an exhaustive, comprehensive and carefully curated source…
