Press release
In vitro ADME testing services market is likely to be worth USD 2.2 billion by 2030, growing at an annualized rate of ~9.8%, predicts Roots Analysis
With increasing cases of drug failure, due to problems associated with pharmacokinetic profiles of candidate therapies, absorption, distribution, metabolism and excretion (ADME) properties and inherent toxicity, industry players are actively looking for more advanced solutions.Roots Analysis has announced the addition of “In Vitro ADME Testing Services Market, 2019-2030” report to its list of offerings.
According to the US Food and Drug Administration (USFDA), less than 10% of investigational new drug (IND) candidates progress beyond the submission of a new drug application (NDA); this implies that majority of the drug / therapy candidates fail to reach the market owing to unacceptable safety and efficacy profiles and the problems associated with their pharmacokinetic profiles, ADME properties and inherent toxicity. ADME studies are considered to be critical in establishing the safety and efficacy of drug candidates.
To order this 280+ page report, which features 135+ figures and 120+ tables, please visit this link- https://www.rootsanalysis.com/reports/view_document/in-vitro-adme-testing-services-market-2019-2030/239.html
Key Market Insights
Nearly 100 players currently claim to provide in vitro ADME testing services
Nearly 60% of these companies are small and mid-sized firms. Further, close to 35% of the CROs engaged in this domain, claim to provide services to both pharmaceutical companies and academic institutes.
Nearly 80% of in vitro ADME testing service providers are based in the developed geographies
Within North America, the US has the maximum number of players, whereas, in Europe, most of the service providers are distributed across France, Germany, the UK, and Spain. On the other hand, there are companies that are using this approach for drug discovery operations, in emerging regions, such as Australia, India and China, as well.
Over 85% companies claim to offer assays for drug metabolism and elimination testing
Further, nearly 70% companies claim to have capabilities to conduct absorption and distribution related studies, respectively. It is worth mentioning that about 35% of players presently offer end-to-end drug discovery services.
Nearly 95% CROs have received operational approval and certification from the USFDA
In addition, companies have received necessary certifications from the EMA (50%), MHLW / PMDA (15%), ICH (13%), WHO (11%), MHRA (11%), followed by CFDA / MFDS / SFDA (9%) and TGA (4%).
Over 35 acquisitions have taken place amongst various stakeholders, between 2005-2018
The addition of capabilities (primarily related to drug metabolism and pharmacokinetics testing) emerged as the most important value drivers across all the acquisitions. Other key value drivers include geographical consolidation and geographical expansion.
North America and Europe are anticipated to capture over 70% of the market share by 2030
Within North America, US is anticipated to hold the 90% of the market share. It is worth mentioning that the market in Asia-Pacific region is anticipated to grow at a relatively faster rate (~11%).
To request a sample copy / brochure of this report, please visit this link - https://www.rootsanalysis.com/reports/view_document/in-vitro-adme-testing-services-market-2019-2030/239.html
Key Questions Answered
Who are the leading CROs offering in vitro ADME testing services?
What are the key services being offered by in vitro ADME testing service providers?
What is the trend of mergers and acquisitions in this domain?
How is the current and future market opportunity likely to be distributed across key market segments?
What are the anticipated future trends related to in vitro ADME testing services market?
The USD 2.2 billion (by 2030) financial opportunity within the vitro ADME testing services market has been analyzed across the following segments:
Type of Molecule
Small Molecules
Biologics
Type of Service
Absorption Testing
Distribution Testing
Metabolism and Elimination Testing
Type of Assay
Caco-2 Permeability Assay
PAMPA Permeability Assay
MDCK Permeability Assay
Protein Binding Assay
Blood Brain Barrier Assay
Blood to Plasma Ratio
Stability / Clearance Assay
Enzyme Induction Assay
Enzyme Inhibition Assay
Metabolite Profiling and Screening / Identification Assay
Metabolite Production Assay
Reaction Phenotyping Assay
Transporter Interactions Assay
Target Therapeutic Area
Blood Disorders
Cardiovascular Disorders
Gastrointestinal and Digestive Disorders
Hormonal Disorders
Infectious Diseases
Immunological Disorders
Metabolic Disorders
Mental Disorders
Neurological Disorders
Oncological Disorders
Respiratory Disorders
Skin Disorders
Urogenital Disorders
Others
Type of Sponsor
Industry Players
Non-Industry Players
Key Geographical Regions
North America
Europe
Asia-Pacific and Rest of the World
The report also features inputs from a number of eminent industry stakeholders. In fact, one of the experts interviewed concurred on the opinion that the drug developers today, prefer to opt for contract service providers that offer a range of capabilities, such as design, synthesis, initial scale-up, in vitro ADME testing, safety pharmacology, under one roof; this guarantees a certain degree of ease of operation, and enables sponsors to shortlist and rely on a capable partner for their outsourcing requirements.” The report features detailed transcripts of discussions held with the following individuals:
Dan Close (Chief Scientific Officer, 490 Bio Tech)
Sridhar Iyer (Director and Global Head, Business Development, JRF Global) and Sarang Gorte (Assistant Manager, Business Development, JRF Global)
The research covers detailed profiles and assesses product portfolios of several companies, including (illustrative list, no selection criteria):
Albany Molecular Research (AMRI)
Charles River Laboratories
Pharmaceutical Product Development (PPD)
RTI International
Eurofins Scientific
Evotec
Galapagos
Tecan Group
GVK Biosciences
Pharmaron
Sai Life Sciences
Shanghai Medicilon
WuXi AppTec
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/in-vitro-adme-testing-services-market-2019-2030/239.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. In Silico / Computer-Aided Drug Discovery Services Market: Focus on Large Molecules, 2020-2030
2. AR / VR based Healthcare Digital Marketing Service Providers Market, 2020-2030
3. Bioavailability Enhancement Technologies and Services Market, 2020-2030
Contact:
Gaurav Chaudhary
gaurav.chaudhary@rootsanalysis.com
Roots Analysis
A430, 4th Floor,
Bestech Business Towers, Sector 66, Mohali, India
sales@rootsanalysis.com
+1 (415) 800 3415
+44 (122) 391 1091
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release In vitro ADME testing services market is likely to be worth USD 2.2 billion by 2030, growing at an annualized rate of ~9.8%, predicts Roots Analysis here
News-ID: 2210726 • Views: …
More Releases from Roots Analysis
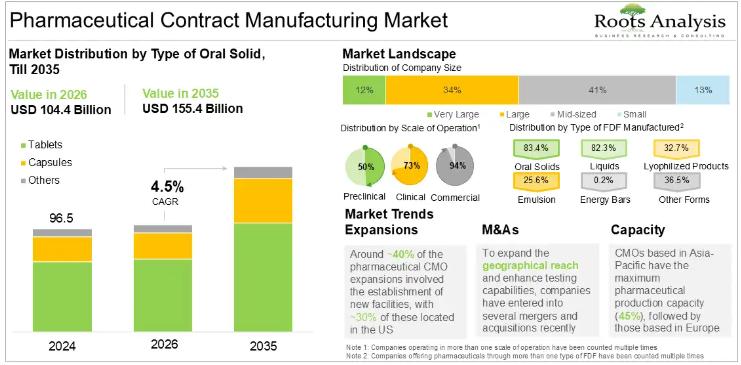
Pharmaceutical Contract Manufacturing Market CAGR To Reach 4.5% between 2025 and …
According to our latest market report "Pharmaceutical Contract Manufacturing Market by Type of Product Manufactured, Type of API, API Potency, Type of FDF, Dosage Form, Type of Oral Solid, Type of Packaging Offered, Scale of Operation, End User, Geographical Regions and Key Players: Industry Trends and Global Forecasts, till 2035", the pharmaceutical contract manufacturing market is estimated to be USD 100.3 billion in 2025. It is expected to reach USD…
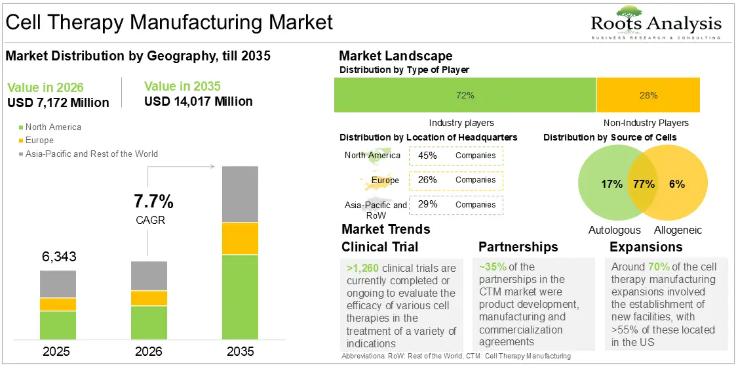
Cell Therapy Manufacturing Market CAGR To Exceed 8.25% by 2035, Due to the Growi …
According to our latest market report "Cell Therapy Manufacturing Market by Type of Cell Therapy, Source of Cells, Scale of Operation, Type of Manufacturer and Key Geographical Regions: Industry Trends and Global Forecasts, 2023-2035", the global cell therapy manufacturing market size is projected to reach USD 14,017 million by 2035 from USD 6,343 million in 2025, growing at a CAGR of 8.25% in the forecast period 2025-2035.
To request quote…
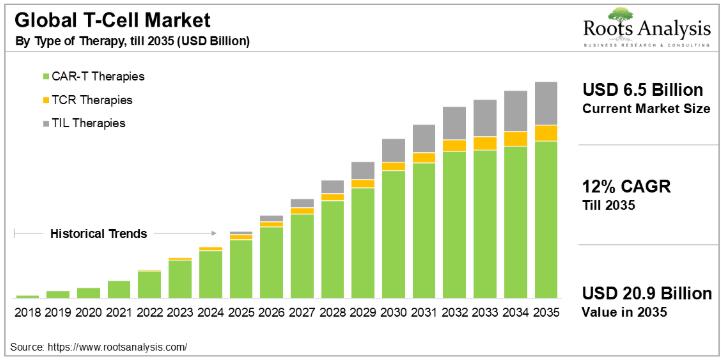
T-Cell Therapy Market Size to Hit USD 20.9 billion by 2035| Exclusive Report by …
Cancer is one of the leading causes of mortality across the world. As per the International Agency for Research on Cancer (IARC), by 2040, there are likely to be 27.5 million new cases and 16.3 million deaths related to cancer, annually. Although cancer therapeutics continue to be one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. In fact,…
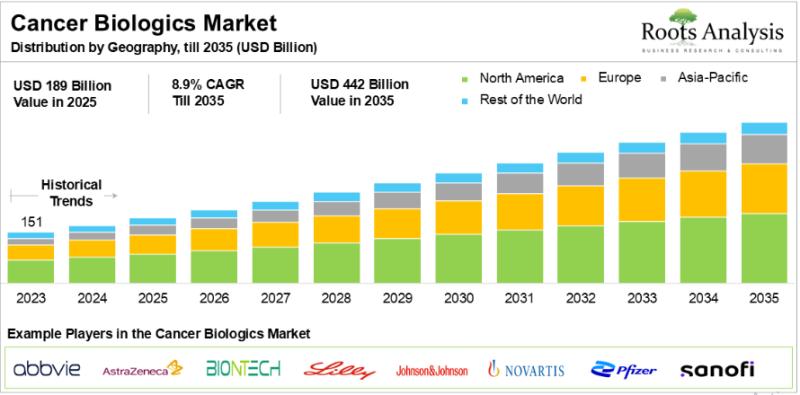
Cancer Biologics Market: Unmet Need and Treatment Guidelines
Owing to the increasing mortality rates and growing need for novel modalities to treat oncological disorders, several researchers and industry stakeholders have shifted their focus on the development of safe and effective biologic therapies. Cancer biologics are the class of therapeutic agents, which primarily modulate immune responses or directly inhibits oncogenic pathways in malignancies. These therapies, such as monoclonal antibodies, specifically target tumor-activating genes, facilitate antibody-dependent cellular cytotoxicity and complement…
More Releases for ADME
ADME Toxicology Testing Market Trends That Will Shape the Next Decade: Insights …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the ADME Toxicology Testing Market Size By 2025?
There has been a swift expansion in the market size of ADME toxicology testing in the past few years. The market, which is projected to surge from $10.4 billion in 2024 to $11.53 billion in 2025, is expected…
Radiolabelled ADME Studies Market Outlook and Future Projections for 2030
The radiolabelled adme studies market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
ADME Toxicology Testing Market Know the Scope and Trends
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "ADME Toxicology Testing Market"- By Type of Services (Absorption, Distribution, Metabolism, Excretion), Type of Assays(Batch / Fed-Batch, Continuous), Type of Molecule (Biologics, Small Molecules), End User(Pharmaceutical and Biotechnology Companies, Academic / Research Institutes), Therapeutic Areas(Blood Disorder, Cardiovascular Disorder, Gastrointestinal and Vascular Disorder, Hormonal Disorder, Infectious Diseases, Immunological Disorders, Mental Disorders, Metabolic Disorders, Neurological Disorders, Oncology…
Global ADME Toxicology Testing Market Insights and Forecast 2024
"The Business Research Company recently released a comprehensive report on the Global ADME Toxicology Testing Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive…
Radiolabelled ADME Studies Market Outlook and Future Projections for 2030
The radiolabelled adme studies market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
ADME Toxicology Testing Market Strategies 2024-2033 | Size, Insights, Demand, Ou …
"The new report published by The Business Research Company, titled ADME Toxicology Testing Global Market Report 2024 - Market Size, Trends, And Global Forecast 2024-2033, delivers an in-depth analysis of the leading size and forecasts, investment opportunities, winning strategies, market drivers and trends, competitive landscape, and evolving market trends.
As per the report, the adme toxicology testing market size has grown rapidly in recent years. It will grow from $9.3…
