Press release
Growing at an annualized rate of over 16.5%, the cell therapy manufacturing market is estimated to reach close to USD 11 Billion by 2030, claims Roots Analysis
The approval of KYMIRAH®, YESCARTA®, Alofisel® and Zyntelgo® has increased the interest of pharma stakeholders in cell therapies; further, owing to the technical challenges in this field, outsourcing manufacturing operations has become a necessityRoots Analysis has announced the addition of “Cell Therapy Manufacturing Market (3rd Edition), 2019 - 2030” report to its list of offerings.
Owing to various reasons, the demand for cell therapies is anticipated to increase over the coming years. Therefore, both therapy developers and contract service providers may need to strengthen their capabilities and expand available capacity. In this context, automation is expected to be a key enabler within the cell therapy manufacturing and contract services industry.
To order this 550+ page report, which features 160+ figures and 250+ tables, please visit this link https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html
Key Market Insights
More than 160 organizations claim to be engaged in cell therapy manufacturing
The market landscape is dominated by industry players, representing more than 60% of the total number of stakeholders. Amongst these, over 55 are large or mid-sized firms (having more than 50 employees).
100+ players focused on T-cell and stem cell therapies
Most of these players are focused on manufacturing T-cell therapies, including CART, TCR or TILs. It is worth highlighting that more than 35 organizations claim to have necessary capabilities for the manufacturing of both types of therapies.
Presently, 70+ companies have commercial scale capacity
As majority of the cell therapy products are in clinical trials, the demand is high at this scale. However, it is worth noting that several players (~50%) have already developed commercial scale capacity for cell therapies.
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
Europe is currently considered a current hub for cell therapy production
More than 220 manufacturing facilities have been established by various players, worldwide; of these, 35% are in Europe, followed by those based in North America. Other emerging regions include Australia, China, Japan, Singapore, South Korea and Israel.
50+ facility expansions reported between 2015-2019
More than 85% of the expansions are related to setting up of new facilities across different regions. Maximum expansion activity was observed in the US and in certain countries within the Asia Pacific regions.
20+ companies offer automated solutions to cell therapy developers
Players that claim to offer consultancy services related to automation include (in alphabetical order) Berkeley Lights, Cesca Therapeutics, Ferrologix, FluDesign Sonics, GE Healthcare and Terumo BCT. Further, we identified players, namely (in alphabetical order) Fraunhofer Institute for Manufacturing Engineering and Automation IPA, Invetech, KMC Systems, Mayo Clinic Center for Regenerative Medicine and RoosterBio, that offer consultancy solutions related to automation.
Partnership activity has grown at an annualized rate of 16%, between 2014 and 2018
More than 200 agreements have been inked in the last 5 years; majority of these were focused on the supply of cell-based therapy products for clinical trials. Other popular types of collaboration models include manufacturing process development agreements (16%), services agreements (12%) and acquisitions (10%).
By 2030, developed geographies will capture over 60% of the market share
Asia Pacific is anticipated to capture the major share (~36%) of the market by 2030. It is also important to highlight that financial resources, technical expertise and established infrastructure is likely to drive cell therapy manufacturing market in Europe, which is estimated to grow at a CAGR of ~26%.
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
Key Questions Answered
What is the global demand for cell-based therapies?
Who are the key manufacturers (industry / non-industry) of cell-based therapies, across the world?
What are the major recent developments (such as partnerships and expansions) in this industry?
What kind of partnership models are commonly adopted by stakeholders in this domain?
What is the current, installed contract manufacturing capacity for cell therapies?
What are the key factors influencing the make (manufacture in-house) versus buy (outsource) decision related to cell therapies?
What are the key parameters governing the cost of cell therapy manufacturing?
What are important technology platforms (available / under development) for cell therapy development and manufacturing?
What are the key drivers and growth constraints in cell therapy manufacturing market?
How is the current and future market opportunity likely to be distributed across key market segments?
The USD 11 billion (by 2030) financial opportunity within the cell therapy manufacturing market has been analyzed across the following segments:
Type of therapy
T-cell therapies (CAR-T therapies, TCR therapies, TIL therapies)
Dendritic cell therapies
Tumor cell therapies
NK cell therapies
Stem cell therapies
Source of cells
Autologous
Allogeneic
Scale of operation
Clinical
Commercial
Purpose of manufacturing
Contract manufacturing
In-house manufacturing
Key geographical regions
North America
Europe
Asia Pacific
Rest of the world
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
The report features inputs from eminent industry stakeholders, according to whom the manufacturing of cell therapies is largely being outsourced due to exorbitant costs associated with the setting-up of in-house expertise. The report includes detailed transcripts of discussions held with the following experts:
Victor Lietao Li (Co-Founder and Chief Executive Officer, Lion TCR)
Tim Oldham (Chief Executive Officer, Cell Therapies)
Gerard MJ Bos (Chief Executive Officer, CiMaas)
Wei (William) Cao (Chief Executive Officer, Gracell Biotechnologies)
Troels Jordansen (Chief Executive Officer, Glycostem Therapeutics)
Arik Hasson (Executive VP Research and Development, Kadimastem)
Gilles Devillers (General Manager, Bio Elpida)
Arnaud Deladeriere (Manager, Business Development & Operations-cGMP Manufacturing Unit, Center of Excellence for Cellular Therapy / C3i)
Brian Dattilo (Manager of Business Development, Waisman Biomanufacturing)
Fiona Bellot (Business Development Manager, RoslinCT)
Mathilde Girard (Department Leader, Cell Therapy Innovation and Development, YposKesi)
David Mckenna (Professor and American Red Cross Chair in Transfusion Medicine, University of Minnesota)
The research covers profiles of key players (industry and non-industry) that offer manufacturing services for cell-based therapies, featuring a company overview, information on manufacturing facilities, and recent collaborations.
BioNTech Innovative Manufacturing Services
Cell Therapies
Cell and Gene Therapy Catapult
Center for Cell and Gene Therapy, Baylor College of Medicine
Centre for Cell Manufacturing Ireland, National University of Ireland
Clinical Cell and Vaccine Production Facility, University of Pennsylvania
Cognate BioServices
FUJIFILM
Guy’s and St. Thomas’ GMP Facility, Guy’s Hospital
Hitachi Chemical
KBI Biopharma
Laboratory for Cell and Gene Medicine, Stanford University
Lonza
MaSTherCell
MEDINET
Molecular and Cellular Therapeutics, University of Minnesota
Newcastle Cellular Therapies Facility, Newcastle University
Nikon CeLL innovation
Rayne Cell Therapy Suite, King’s College London
Roslin Cell Therapies
Scottish National Blood Transfusion Services Cellular Therapy Facility, Scottish Centre for Regenerative Medicine
Sydney Cell and Gene Therapy
WuXi Advanced Therapies
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Cell and Advanced Therapies Supply Chain Management Market, 2019-2030
2. RNAi Therapeutics Market (2nd Edition), 2019 – 2030
3. Gene Therapy Market (3rd Edition), 2019 – 2030
4. Stem Cell Therapy Contract Manufacturing Market, 2019-2030
Contact:
Gaurav Chaudhary
+1 (415) 800 3415
Gaurav.Chaudhary@rootsanalysis.com
Contact:
Gaurav Chaudhary
gaurav.chaudhary@rootsanalysis.com
Roots Analysis
A430, 4th Floor,
Bestech Business Towers, Sector 66, Mohali, India
sales@rootsanalysis.com
+1 (415) 800 3415
+44 (122) 391 1091
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Growing at an annualized rate of over 16.5%, the cell therapy manufacturing market is estimated to reach close to USD 11 Billion by 2030, claims Roots Analysis here
News-ID: 2195845 • Views: …
More Releases from Roots Analysis
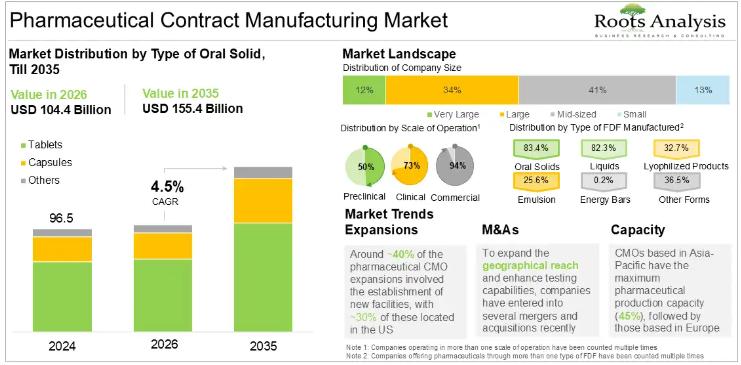
Pharmaceutical Contract Manufacturing Market CAGR To Reach 4.5% between 2025 and …
According to our latest market report "Pharmaceutical Contract Manufacturing Market by Type of Product Manufactured, Type of API, API Potency, Type of FDF, Dosage Form, Type of Oral Solid, Type of Packaging Offered, Scale of Operation, End User, Geographical Regions and Key Players: Industry Trends and Global Forecasts, till 2035", the pharmaceutical contract manufacturing market is estimated to be USD 100.3 billion in 2025. It is expected to reach USD…
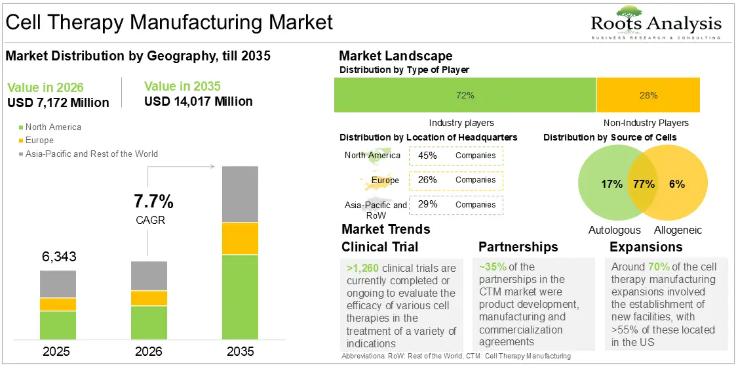
Cell Therapy Manufacturing Market CAGR To Exceed 8.25% by 2035, Due to the Growi …
According to our latest market report "Cell Therapy Manufacturing Market by Type of Cell Therapy, Source of Cells, Scale of Operation, Type of Manufacturer and Key Geographical Regions: Industry Trends and Global Forecasts, 2023-2035", the global cell therapy manufacturing market size is projected to reach USD 14,017 million by 2035 from USD 6,343 million in 2025, growing at a CAGR of 8.25% in the forecast period 2025-2035.
To request quote…
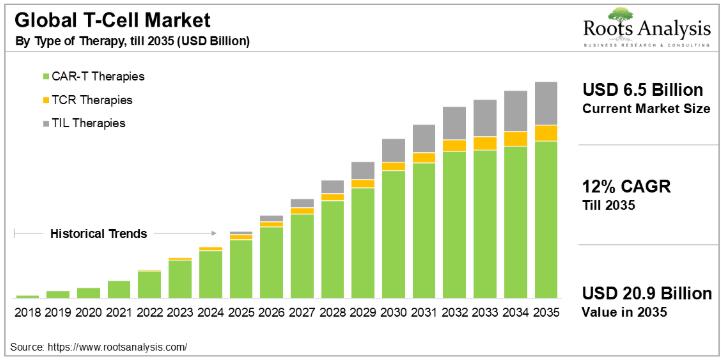
T-Cell Therapy Market Size to Hit USD 20.9 billion by 2035| Exclusive Report by …
Cancer is one of the leading causes of mortality across the world. As per the International Agency for Research on Cancer (IARC), by 2040, there are likely to be 27.5 million new cases and 16.3 million deaths related to cancer, annually. Although cancer therapeutics continue to be one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. In fact,…
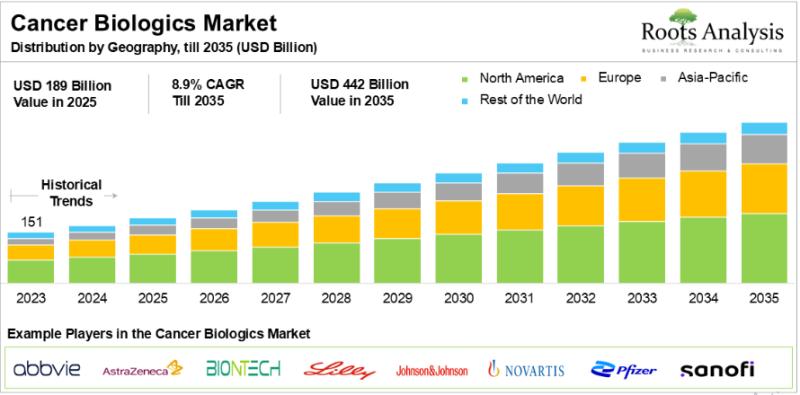
Cancer Biologics Market: Unmet Need and Treatment Guidelines
Owing to the increasing mortality rates and growing need for novel modalities to treat oncological disorders, several researchers and industry stakeholders have shifted their focus on the development of safe and effective biologic therapies. Cancer biologics are the class of therapeutic agents, which primarily modulate immune responses or directly inhibits oncogenic pathways in malignancies. These therapies, such as monoclonal antibodies, specifically target tumor-activating genes, facilitate antibody-dependent cellular cytotoxicity and complement…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…
