Press release
Adoptive Cellular Immunotherapy Market Rising at a High CAGR by Forecast Period 2020-2025 with Top Vendors Like Amgen, Autolus Therapeutics, Beijing Immunochina, Bellicum, Bristol-Myers Squibb
The growing demand for Adoptive Cellular Immunotherapy has provided a major boost to the Global Adoptive Cellular Immunotherapy Market as more people are shifting their preferences to this growing sector. The market is expected to keep rising at a high CAGR and reach values of high millions by the end of the forecast period of 2020 up to 2025.Global Adoptive Cellular Immunotherapy Market overview:
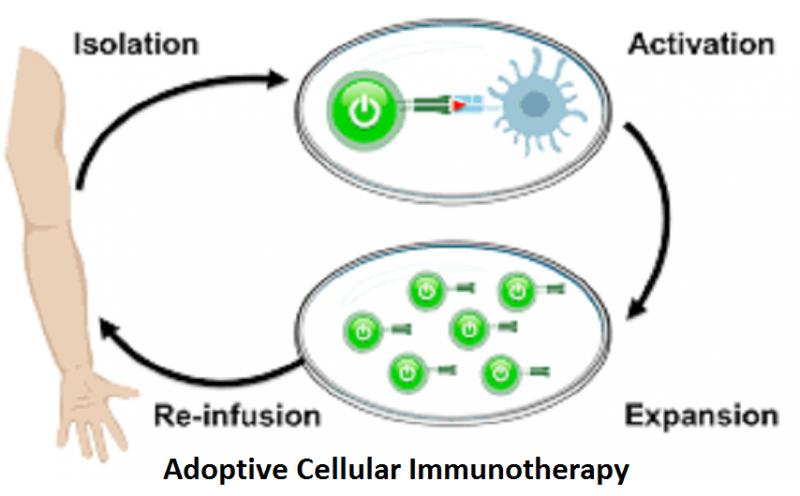
Adoptive cell therapy, also known as cellular immunotherapy, is a form of treatment that uses the cells of our immune system to eliminate cancer. Some of these approaches involve directly isolating our own immune cells and simply expanding their numbers, whereas others involve genetically engineering our immune cells (via gene therapy) to enhance their cancer-fighting capabilities. The Global Adoptive Cellular Immunotherapy Market is segmented on the basis of Product Type, Application, End Use Industry and Region. This report also covers all the regions and countries of the world, which shows a regional development status, including market size.
Our immune system is capable of recognizing and eliminating cells that have become infected or damaged as well as those that have become cancerous. In the case of cancer, immune cells known as killer T cells are particularly powerful against cancer, due to their ability to bind to markers known as antigens on the surface of cancer cells.
Available Exclusive Sample Copy of this Report @ https://www.businessindustryreports.com/sample-request/280063 .
On the Basis of Product Type segment, the Adoptive Cellular Immunotherapy Market is sub segmented into TIL, LAK, CAR-T, TCR-T, CIK, NK, DC and Other. Based on End Use Industry segment, the Adoptive Cellular Immunotherapy Market is segmented as Hospital, Cancer Hospital, Centers for Disease Control and Prevention, Rehabilitation Center and Other.
There are several manufacturers of Adoptive Cellular Immunotherapy in Europe and North America. In North America, the demand for Adoptive Cellular Immunotherapy is primarily driven by the Healthcare sector. Among the countries in Asia Pacific, the demand was substantially high in developing countries such as China and India.
Purchase this report online with 90 Pages, List of Tables & Figures and in-depth Table of Contents on “Adoptive Cellular Immunotherapy Market Report 2020” @ https://www.businessindustryreports.com/buy-now/280063/single .
Top Industry News:
Amgen Announces Participation Of Systemic Lupus Erythematosus (SLE) Adaptive Clinical Trial In The FDA Complex Innovative Trial Designs (CID) Pilot Program on 27 October, 2020 -- Amgen today announced that it has completed trial design discussions through two Complex Innovative Trial Designs (CID) Pilot Program meetings with the U.S. Food and Drug Administration (FDA) for its planned Phase 2 efficacy and safety trial for efavaleukin alfa (formerly known as AMG 592), an investigational candidate for Systemic Lupus Erythematosus (SLE) treatment. The CID Pilot Program aims to modernize drug development, improve efficiency, and promote innovation. The efavaleukin alfa participation in the CID Pilot Program is based on an innovative adaptive clinical trial design developed to foster the acceleration of a potential therapeutic option that could benefit patients living with SLE.
"Systemic Lupus Erythematosus is an area with significant need for new therapies for those living with the condition, but one that has been challenging to address given the complexity of this autoimmune disease," said Rob Lenz, M.D., Ph.D., senior vice president, Global Development at Amgen. "Our partnership with the FDA on the CID Pilot Program should drive the development of a new treatment for lupus to address unmet need for patients."
"Amgen welcomes the opportunity to partner with the FDA through participation in the CID Pilot Program, which intends to advocate innovative clinical trial designs, as well as provide the FDA an opportunity to communicate these advances publicly," said Steven Galson, M.D., senior vice president, Global Regulatory Affairs and Strategy at Amgen. "We appreciate the FDA's efforts, significant contributions and feedback provided throughout the Pilot process."
The CID Pilot Program fulfills a performance goal agreed to under the Prescription Drug User Fee Act (PDUFA) VI, aiming to facilitate and advance the use of novel clinical trial designs that support the development and regulatory review of new therapeutics. Designs under the CID umbrella include, but are not limited to, complex adaptive, Bayesian, and other novel clinical trial designs which often require simulations to determine the statistical properties of the trial. The FDA considers several eligibility factors when selecting qualifying programs, including the level of innovation of the trial design, and the therapeutic need.
Grab Your Report at an Impressive Discount @ https://www.businessindustryreports.com/check-discount/280063 .
Major Key Players:
Some of the Adoptive Cellular Immunotherapy Market manufacturers involved in the market are Amgen Inc, Autolus Therapeutics Plc, Beijing Immunochina Medical Science & Technology Co Ltd, Bellicum Pharmaceuticals Inc, Bristol-Myers Squibb Co, bluebird bio Inc, CARsgen Therapeutics Ltd, Celgene Corp, Cell Medica Ltd, Cellular Biomedicine Group Inc, Celularity Inc, Celyad SA, Daiichi Sankyo Co Ltd, Fosun Pharmaceutical AG, Gilead Sciences Inc, Guangzhou Anjie Biomedical Technology Co Ltd, Hangzhou Converd Co Ltd, Hebei Senlang Biotechnology Inc Ltd, HRAIN Biotechnology Co Ltd, Juno Therapeutics Inc, Kite Pharma Inc, Nanjing Legend Biotech Co Ltd, NantKwest Inc, Nkarta Inc, Novartis AG, Ono Pharmaceutical Co Ltd, Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd, Sorrento Therapeutics Inc, Takara Bio Inc in World, effective mergers are some of the strategies adopted by the Adoptive Cellular Immunotherapy Market manufacturers. New product launches and continuous technological innovations are the Adoptive Cellular Immunotherapy Market strategies adopted by the major players.
Table of Contents:
1 Adoptive Cellular Immunotherapy Product Definition
2 Global Adoptive Cellular Immunotherapy Market Manufacturer Share and Market Overview
2.1 Global Manufacturer Adoptive Cellular Immunotherapy Shipments
2.2 Global Manufacturer Adoptive Cellular Immunotherapy Business Revenue
2.3 Global Adoptive Cellular Immunotherapy Market Overview
3 Manufacturer Adoptive Cellular Immunotherapy Business Introduction
3.1 Amgen Inc Adoptive Cellular Immunotherapy Business Introduction
3.1.1 Amgen Inc Adoptive Cellular Immunotherapy Shipments, Price, Revenue and Gross profit 2020-2025
3.1.2 Amgen Inc Adoptive Cellular Immunotherapy Business Distribution by Region
3.1.3 Amgen Inc Interview Record
3.1.4 Amgen Inc Adoptive Cellular Immunotherapy Business Profile
3.1.5 Amgen Inc Adoptive Cellular Immunotherapy Product Specification
3.2 Autolus Therapeutics Plc Adoptive Cellular Immunotherapy Business Introduction
3.2.1 Autolus Therapeutics Plc Adoptive Cellular Immunotherapy Shipments, Price, Revenue and Gross profit 2020-2025
3.2.2 Autolus Therapeutics Plc Adoptive Cellular Immunotherapy Business Distribution by Region
3.2.3 Interview Record
3.2.4 Autolus Therapeutics Plc Adoptive Cellular Immunotherapy Business Overview
3.2.5 Autolus Therapeutics Plc Adoptive Cellular Immunotherapy Product Specification
3.3 BEIJING IMMUNOCHINA MEDICAL SCIENCE & TECHNOLOGY CO LTD Adoptive Cellular Immunotherapy Business Introduction
3.3.1 BEIJING IMMUNOCHINA MEDICAL SCIENCE & TECHNOLOGY CO LTD Adoptive Cellular Immunotherapy Shipments, Price, Revenue and Gross profit 2020-2025
3.3.2 BEIJING IMMUNOCHINA MEDICAL SCIENCE & TECHNOLOGY CO LTD Adoptive Cellular Immunotherapy Business Distribution by Region
3.3.3 Interview Record
3.3.4 BEIJING IMMUNOCHINA MEDICAL SCIENCE & TECHNOLOGY CO LTD Adoptive Cellular Immunotherapy Business Overview
3.3.5 BEIJING IMMUNOCHINA MEDICAL SCIENCE & TECHNOLOGY CO LTD Adoptive Cellular Immunotherapy Product Specification
3.4 Bellicum Pharmaceuticals Inc Adoptive Cellular Immunotherapy Business Introduction
3.5 Bristol-Myers Squibb Co Adoptive Cellular Immunotherapy Business Introduction
3.6 Bluebird bio Inc Adoptive Cellular Immunotherapy Business Introduction
About us
BusinessindustryReports.com is digital database of comprehensive market reports for global industries. As a market research company, we take pride in equipping our clients with insights and data that holds the power to truly make a difference to their business. Our mission is singular and well-defined – we want to help our clients envisage their business environment so that they are able to make informed, strategic and therefore successful decisions for themselves.
Media Contact
Business Industry Reports
Pune – India
sales@businessindustryreports.com
+19376349940
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Adoptive Cellular Immunotherapy Market Rising at a High CAGR by Forecast Period 2020-2025 with Top Vendors Like Amgen, Autolus Therapeutics, Beijing Immunochina, Bellicum, Bristol-Myers Squibb here
News-ID: 2177470 • Views: …
More Releases from Business Industry Reports
Tetracaine Market Exhibits a Lucrative Growth Potential during 2021-2025 | Endo …
BusinessIndustryReports has recently broadcasted a new study to its broad research portfolio, which is titled as “Global Tetracaine Market” Research Report 2021 provides an in-depth analysis of the Tetracaine with the forecast of market size and growth. The analysis includes addressable market, market by volume, market share by business type and by segment (external and in-house). The research study examines the Tetracaine on the basis of a number of criteria,…

Cyber Warfare Market Evenly Poised To Reach A Market Value of US$ By Share, Size …
Overview of Global Cyber Warfare Market:
This report provides in-depth study of “Global Cyber Warfare Market 2021” using SWOT analysis i.e. Strength, Weakness, Opportunities, and Threat to the organization. The Cyber Warfare Market report also provides an in-depth survey of key players in the market organization.
According to the market research study, the Cyber Warfare is virtual conflict between state, organization, or country by the use of computer technology to disrupt activities…
Covid-19 Impact on Cranio Maxillofacial Implant Market 2021-2025: Business Growt …
Overview of Global Cranio Maxillofacial Implant Market:
This report provides in-depth study of “Global Cranio Maxillofacial Implant Market 2021” using SWOT analysis i.e. Strength, Weakness, Opportunities, and Threat to the organization. The Cranio Maxillofacial Implant Market report also provides an in-depth survey of key players in the market organization.
According to the market research study, Craniomaxillofacial Implants are medical implants used in surgeries of maxillofacial region such as, head, face, neck, oral,…

Beginning of the bloom: The Rise of the Biohacking Market 2021-2025 | Global Key …
Global Biohacking Market Synopsis:
The report covers a forecast and an analysis of the Biohacking Market on a global and regional level. The study provides historical data for 2015, 2016, 2017 and 2018 along with a forecast from 2020 to 2025 based on revenue (USD Million) and volume (Kilotons). The study includes drivers and restraints of the Biohacking Market along with the impact they have on the demand over the forecast…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…