Press release
Keratoconus Market Trends, Key Players Insight, Epidemiology and Market Forecast to 2030
Keratoconus (KC) Market Insights, Epidemiology and Market Forecast, 2030 report delivers an in-depth understanding of the Keratoconus (KC), historical and forecasted epidemiology as well as the Keratoconus (KC) market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.The Keratoconus (KC) market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Keratoconus (KC) market size from 2017 to 2030. The report also covers current Keratoconus (KC) treatment practice/algorithm, market drivers, market barriers and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
Geography Covered
- The United States
- EU5 (Germany, France, Italy, Spain, and the United Kingdom)
- Japan
Study Period: 2017-2030
Get Free Sample Copy of this Research Report at https://www.reportsnreports.com/contacts/requestsample.aspx?name=2888982
Keratoconus (KC) Disease Understanding and Treatment Algorithm
Keratoconus (KC) Overview
Keratoconus (KC) also known as the conical cornea, is an eye (ocular) disorder characterized by progressive thinning and changes in the shape of the cornea. The cornea is the thin, clear outer layer of the eye and is normally dome-shaped. Slowly progressive thinning of the cornea causes a cone-shaped bulge to develop towards the center of the cornea in the areas of greatest thinning. Affected individuals develop a blurry or distorted vision, sensitivity to light (photophobia), and additional vision problems. KC has different types based on the shape and location of the thinned cornea. These types include nipple, oval, keratoglobus, and D-shaped KC.
Generally, KC is first diagnosed in young people at puberty or in their late teens. The exact cause of KC is unknown. There are many theories based on research and its association with other conditions such as allergies and genetic causes; however, no one theory explains it all, and it may be caused by a combination of things.
In its earliest stages, KC is known to cause slight blurring and distortion of vision and increased sensitivity to glare and light; these symptoms usually appear in the late teens or early 20s. It may progress for 1020 years and then slow in its progression. Each eye may be affected differently.
The specific underlying mechanism(s) responsible for KC are not fully understood. Most cases appear to occur randomly for unknown reasons (sporadically). However, a positive family history of KC has been established in some cases. Most researchers believe that multiple, complex factors are required for the development of KC, including both genetic and environmental factors.
Get 20% Discount on this Research Report at https://www.reportsnreports.com/contacts/discount.aspx?name=2888982
Keratoconus (KC) Diagnosis
KC should be suspected in any patient with significant irregular astigmatism, especially if unstable and increasing over time. In the early stages of the disease, there is altered metabolic activity that may lead to biomechanical instability and stretching of the corneal tissues. As the disease progresses, there is accompanying tissue loss. In addition, there is a loss of correlation between the anterior and posterior corneal curvature. Progressive corneal thinning and distortion causes a conical or cone-shaped protrusion, which may be visible at the slit lamp in advanced cases. In early disease, the condition may go undiagnosed unless assessments of the posterior and anterior corneal surfaces are undertaken using corneal tomography.
KC may be diagnosed based upon a complete patient and family history and thorough eye examination. Such an examination may include evaluation of the external appearance of the eyes, visual acuity, eye movements, and visual fields; the use of a special, illuminated microscope that allows physicians to view the eye through high magnification (slit-lamp examination); and/or additional tests or procedures.
Various diagnostic techniques and procedures used for KC include:
- Eye Refraction
- Slit-lamp examination
- Keratometry
- Corneal Topography
- Corneal Tomography
- Keratoconus indices
- The Belin Ambrosio Enhanced Ectasia Display (BAD)
- Holladay 6 map display
- Corneal pachymetry
- Automated detection program for subclinical KC
- Corneal biomechanics
Keratoconus (KC) Treatment
The initial treatment for keratoconus often consists of hard contact lenses. A variety of keratorefractive procedures have also been attempted, broadly divided into subtractive and additive techniques. Subtractive techniques include photorefractive keratectomy or laser in situ keratomileusis, although generally, the results of these techniques have been poor. Implantation of intrastromal corneal ring segments is an additive technique in which the implants are intended to reinforce the cornea, prevent further deterioration, and potentially obviate the need for penetrating keratoplasty. Penetrating keratoplasty (i.e., corneal grafting) is the last line of treatment. About 20% of patients with keratoconus will require corneal transplantation. All of these treatments attempt to improve refractive errors but are not disease-modifying.
Direct Purchase of this Research Report at https://www.reportsnreports.com/purchase.aspx?name=2888982
However, keratoconus management has significantly changed over the last two decades. The advent of new interventions such as cornea cross-linking, intrastromal corneal ring segments and combined treatments provide corneal clinicians a variety of treatment options for the visual rehabilitation of keratoconus patients.
Corneal collagen cross-linking (CXL) has the potential to slow the progression of the disease. During CXL, in order to achieve a strengthening effect of corneal tissue and arrest keratoconus progression, the use of riboflavin (vitamin B2) is combined with ultraviolet A (UV-A) irradiation. Riboflavin plays the role of a photosensitizer in the photopolymerization process and when combined with UV-A irradiation increases the formation of intrafibrillar and interfibrillar carbonyl-based collagen covalent bonds through a molecular process that has still not been completely elucidated.
Keratoconus (KC) Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Prevalent Population of Keratoconus (KC), Severity-specific Distribution of Keratoconus (KC) and Age-specific Distribution of Keratoconus (KC) in the 7MM market covering the United States, EU5 countries (Germany, France, Italy, Spain, and United Kingdom) and Japan from 2017 to 2030.
Key Findings
This section provides glimpse of the Keratoconus (KC) epidemiology in the 7MM.
- The total diagnosed prevalent population of KC in the seven major markets was estimated to be 1,602,427 in 2017.
- The diagnosed prevalent cases of KC, in the United States, were found to be 550,047 in 2017.
- In 2017, the mild, moderate and severe type of KC, in the United States, were found to be 121,010, 313,527, and 115,510, respectively.
- It was found that in the United States, the maximum number of cases of KC was found in the age group of 3039 with 181,515 cases in 2017, while the lowest number of cases were found in the age group =60 with 16,501 cases in 2017.
- In the EU5 countries, the diagnosed prevalence of KC was found to be maximum in Germany, with 218,772 cases, followed by Italy with 171,676 cases in 2017. The least number of cases were found in Spain, with 105,463 cases in 2017.
- In Japan, the diagnosed prevalence of KC was anticipated to be 240,591 in 2017.
Country Wise- Keratoconus (KC) Epidemiology
The epidemiology segment also provides the Keratoconus (KC) epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom) and Japan.
Keratoconus (KC) Drug Chapters
The drug chapter segment of the Keratoconus (KC) report encloses the detailed analysis of Keratoconus (KC) marketed drugs and mid and late stage pipeline drugs. It also helps to understand the Keratoconus (KC) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details of each included drug and the latest news and press releases.
Keratoconus (KC) Marketed Drugs
Photrexa Viscous and Photrexa: Glaukos/ Avedro
Photrexa Viscous (riboflavin 5-phosphate in 20% dextran ophthalmic solution) and Photrexa (riboflavin 5-phosphate ophthalmic solution) are photoenhancers indicated for use with the KXL System in corneal collagen cross-linking. The KXL System is an electronic medical device that delivers ultraviolet light (365 nm wavelength) in a circular pattern onto the cornea after riboflavin phosphates ophthalmic solution (Photrexa Viscous and/or Photrexa) has been applied. They are the first and only FDA approved therapeutic treatment of progressive keratoconus (KC) and corneal ectasia following refractive surgery.
Product details in the report
CE Marked Riboflavin Formulations: Avedro/ Glaukos Corporation
Avedros scientifically-developed family of CE marked riboflavin formulationsVibeX Xtra, VibeX Rapid, ParaCel, MedioCROSS TE, MedioCROSS M, MedioCROSS D, and MedioCROSS Hensure rapid diffusion in individual, sterile syringes for all cross-linking needs. These multiple CE marked riboflavin formulations have set the standard to produce the highest quality riboflavin since the introduction of corneal cross-linking. Avedros riboflavin formulations have been used in hundreds of thousands of treatments around the world. The company distributes its products in 62 countries through 33 ophthalmic distributors with 115 sales and service representatives. The products are not for sale in the US.
Product details in the report
Ricrolin: Sooft Italia
Ricrolin is a hypotonic ophthalmic solution containing Ribofl¬avin (0.1%), specifically formulated to allow quick passage of Riboflavin into the corneal stroma, either through the healthy epithelium or after de-epithelization or even by using a low-intensity electrical field applied topically (iontophoresis). This solution, used on the eye, combined with an emitter of ultraviolet (UV-A) rays, is indicated for the conservative parasurgical treatment of the keratoconus and of corneal ectasia diseases. When administering Ricrolin using iontophoresis, the transepithelial riboflavin penetration, aided by the application of a low-intensity electric field, facilitates the corneal cross-linking operation by shortening absorption time, thereby reducing patient discomfort.
Product details in the report
Collagex Riboflavin: Lightmed_OptiMed
Lightmed developed Collagex riboflavin (vitamin B2) family, which is an essential component of any corneal cross-linking procedure. It is considered as the purest and most advanced riboflavin in the market and is redefined as the ultra hi-quality riboflavin solution. It is supplied in a convenient glass syringe applicator and individually packed in sterile pouches available for use in conjunction with LightMed LightLink CXL corneal cross linking system, or as generic formula. The product is exclusively produced and packaged by a Class II GMP accredited Manufacturing Pharmacy under fully aseptic conditions. This Collagex family consists of several products that are distinguished based on their compositions. These products include CollagexIsotonic, CollagexHypotonic, CollagexTrans-Epithelial (TE), CollagexRapid, and CollagexPlus.
Product details in the report
Peschke Riboflavin Solutions: Peschke Meditrade GmbH
Peschke Riboflavin solutions initialize the formation of new collagen cross-links and protect the eye against the UV-A light applied during the corneal cross-linking procedure. These solutions are available in the various formulations, namely, Peschke TE, Peschke M, Peschke D, Peschke H and Peschke L. These Peschke Riboflavin solution formulations are used in conjunction with the PXL Platinum 330 system. The PXL Platinum 330 treatment is intended to induce corneal collagen cross-linking to improve the biomechanical properties of the cornea by strengthening the corneal tissue in the anterior stroma. This cross-linking procedure is useful for treating Corneal Ectasia, Keratoconus, Iatrogenic Ectasia, Pellucid Marginal Degeneration (PMD), and Infectious keratitis.
Product details in the report
Keratoconus (KC) Emerging Drugs
IVMED-80: iVeena Delivery Systems
IVMED-80 is the first twice-daily, eye-drop, non-surgical, non-UV light treatment for medical crosslinking of the cornea. The drug, with its copper-based formulation, is reportedly the first eye drop designed to treat keratoconus (KC) without the need for adjunctive laser treatment or surgical intervention. IVMED-80s mechanism of action centers on enhancing the activity of lysyl oxidase (LOX) the enzyme responsible for corneal collagen crosslinking, and also known to be associated with KC and copper, which is a co-factor of LOX, is the active ingredient of the drug. In February 2019, the company announced the commencement of a 36 patient phase I/IIa pilot clinical study for the treatment of mild and moderate KC (IVMED-80) and presented an abstract of the trial in the 2020 ARVO Annual Meeting.
Product details in the report
Keratoconus (KC) Market Outlook
According to the Cornea Research Foundation of America, Keratoconus (KCN) is a disease characterized by thinning and protrusion of the cornea, resulting in an irregular, conical shape. Irregular astigmatism occurs as the KC progresses, and results in blurred vision, which can be impossible to correct with spectacles. Usually, keratoconus occurs in both eyes, and involves the central cornea with the apex of the cone just below the visual axis. Approximately 50200 of every 100,000 people are afflicted with KC. In the US, a study found a prevalence of 54.5 per 100,000 people.
The current treatment option includes the use of cornea cross-linking, intrastromal corneal ring segments, and other combined treatments, which provide the corneal clinicians a variety of treatment options for the visual rehabilitation of KC patients. In the mildest form of keratoconus, eyeglasses or soft contact lenses are preferred. But as the disease progresses and the cornea thins and becomes increasingly more irregular in shape, glasses and regular soft contact lens designs no longer provide adequate vision correction. The treatment of progressive KC includes corneal collagen cross-linking or CXL, custom soft contact lenses, gas permeable contact lenses, Piggy backing, contact lenses, Hybrid contact lenses, Scleral and semi-scleral lenses, Prosthetic lenses, Topography-guided conductive keratoplasty (CK), and corneal transplant.
In addition to these therapies, the US FDA has approved Avedros Photrexa and Photrexa Viscous photoenhancers indicated for use with the KXL System in corneal collagen cross-linking for the treatment of patients with progressive KC and post-LASIK ectasia. Although the EMA does not approve this therapy; however, it was used in Europe way before its FDA approval in April 2016 as a CE marked product.
Cross-linking is approved by the FDA for halting the progression of KC. Once the condition progresses, FDA has approved Intacs or a cornea transplant as more advanced treatment options. Crosslinking utilizes riboflavin drops (Vitamin B2) and ultraviolet light, which interact with the corneal tissue to create cross-links between corneal proteins. This strengthens the cornea, which is weak in keratoconus, to decrease KC progression.
In addition to standard riboflavin formulations comparable to the US FDA-approved ophthalmic solutions, a variety of modified riboflavin formulations are in use in regions outside the United States and are regulated under the CE Mark in the European Union. These formulations have not been evaluated by the US FDA and are not approved for sale in the United States. These formulations include the Avedros family of CE marked riboflavin (VibeX Xtra, VibeX Rapid, ParaCel, MedioCROSS TE, MedioCROSS M, MedioCROSS D, and MedioCROSS H), Lightmeds Collagex riboflavin (vitamin B2) family of products which includes- CollagexIsotonic, CollagexHypotonic, CollagexTrans-Epithelial (TE), CollagexRapid, and CollagexPlus and Sooft Italias Ricrolin.
Other than these CE marked products that are being marketed in Europe, Peschke Riboflavin is also available in the European market. These solutions are also available in different formulations, namely Peschke TE, Peschke M, Peschke D, Peschke H, and Peschke L.. These Peschke Riboflavin solution formulations are used in conjunction with the PXL Platinum 330 system.
The current pipeline holds a major unmet need for the novel and potential emerging therapy. The emerging emerging-market holds IVMED-80, which is under development by iVeena Delivery Systems to treat KC without the need for adjunctive laser treatment or surgical intervention. The drug is in phase I/II clinical-developmental trial.
Key Findings
This section includes a glimpse of the Keratoconus (KC) 7MM market.
- The market size of KC in the seven major markets was estimated to be USD 2,456 million in 2017.
- The United States accounted for the largest market size of KC in comparison to EU5 (the United Kingdom, Germany, France, Italy, and Spain) and Japan.
- In 2017, among the EU5 countries, Germany had the largest market size with USD 280 million, while Spain had the smallest market size of KC with USD 135 million.
- The Japan KC market accounted for USD 299 million in 2017.
The United States Market Outlook
This section provides the total Keratoconus (KC) market size and market size by therapies in the United States.
EU-5 Market Outlook
The total Keratoconus (KC) market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom are provided in this section.
Japan Market Outlook
The total Keratoconus (KC) market size and market size by therapies in Japan are provided.
Keratoconus (KC) Drugs Uptake
This section focusses on the rate of uptake of the potential drugs recently launched in the Keratoconus (KC) market or expected to get launched in the market during the study period 20172030. The analysis covers Keratoconus (KC) market uptake by drugs; patient uptake by therapies; and sales of each drug.
This helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs and allow the comparison of the drugs on the basis of market share and size which again will be useful in investigating factors important in market uptake and in making financial and regulatory decisions.
Keratoconus (KC) Development Activities
The report provides insights into different therapeutic candidates in phase II, and phase III stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing and patent details for Keratoconus (KC) emerging therapies.
Competitive Intelligence Analysis
We perform competitive and market Intelligence analysis of the Keratoconus (KC) market by using various competitive intelligence tools that include-SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Report
- The report covers the descriptive overview of Keratoconus (KC), explaining its causes, signs and symptoms, pathogenesis and currently available therapies.
- Comprehensive insight has been provided into the Keratoconus (KC) epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for Keratoconus (KC) are provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of Keratoconus (KC) market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM Keratoconus (KC) market.
Report Highlights
- In the coming years, Keratoconus (KC) market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market.
- The companies and academics are working to assess challenges and seek opportunities that could influence Keratoconus (KC) R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition.
- As per DelveInsights analysis, KC, based on its severity, can be categorized into three types, namely, mild, moderate and severe.
- Delvelnsight has also analysed age-specific data of KC, according to which the overall diagnosed population of KC can be segmented into separate age groups, namely,
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
+ 1 888 391 5441
sales@reportsandreports.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Keratoconus Market Trends, Key Players Insight, Epidemiology and Market Forecast to 2030 here
News-ID: 2160272 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
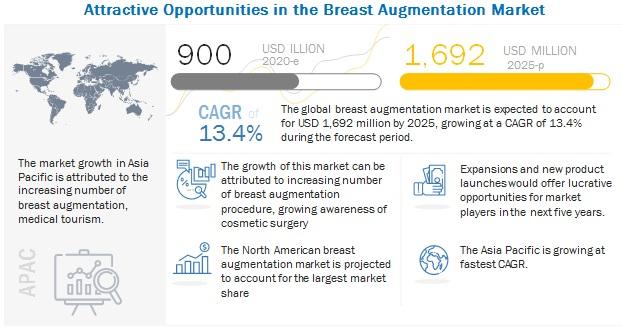
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Keratoconus
Valley Optometry Eyecare Center Brings Advanced Keratoconus Treatment to Reseda …
Image: https://www.globalnewslines.com/uploads/2025/09/1758898268.jpg
Valley Optometry Eyecare Center stands as a cornerstone of comprehensive eye care throughout the Greater Los Angeles region. The practice has earned recognition for combining traditional optometric wisdom with modern diagnostic capabilities. Patients from across the San Fernando Valley have come to depend on this facility for everything from basic vision screenings to complex medical interventions that preserve sight.
Keratoconus presents unique challenges that can leave patients feeling hopeless about…
Valley Optometry Eyecare Broadens Awareness Around Reseda Keratoconus Treatment …
Image: https://www.globalnewslines.com/uploads/2025/08/1754401929.jpg
Valley Optometry Eyecare remains a steady presence in the Los Angeles eye care landscape, recognised for its careful, technology-driven approach to diagnosis and long-term treatment. Operating at the intersection of clinical precision and patient accessibility, the centre continues to address lesser-known but increasingly prevalent conditions affecting local vision health.
Among these concerns, keratoconus stands out for its complexity and the subtlety of its onset. Affecting the shape and strength of…
Valley Optometry Eyecare Provides Breakthrough Keratoconus Therapy
Image: https://www.globalnewslines.com/uploads/2025/05/1748342525.jpg
Valley Optometry Eyecare stands at the forefront of advanced eye care for Southern California residents. The practice operates from its central location with modern equipment and highly trained staff ready to tackle complex vision problems. Eye health specialists at this established center work tirelessly to address conditions many patients fear might permanently affect their sight. Valley Optometry Eyecare has earned trust within the community through consistent results and attention…
Keratoconus Treatment Market Size, Share, Trends, Growth and Competitive Analysi …
"Keratoconus Treatment Market report is an important manuscript for every market enthusiast, policymaker, investor, and market player. A detailed market research study of this report focuses on several essential parameters related to the market that includes but are not limited to a competitive landscape, brief segmentation and industrial infrastructure. This marketing report offers an in-depth overview of product specification, technology, product type and production analysis considering major factors such as…
Keratoconus Market Size, Share, Trends And Forecast To 2034
How big is the keratoconus market?
The keratoconus market reached a value of US$ 202.1 Million in 2023 and expected to reach US$ 275.0 Million by 2034, exhibiting a growth rate (CAGR) of 2.84% during 2024-2034.
The report offers a comprehensive analysis of the keratoconus market in the United States, EU5 (including Germany, Spain, Italy, France, and the United Kingdom), and Japan. It covers aspects such as treatment methods, drugs available in…
Keratoconus Market Size, Growth, Research Report & Trends 2023-2033
Keratoconus Market Report Overview:
Report Attribute Details
Base Year …