Press release
Spinal Muscular Atrophy Pipeline Market Global Insight, Key Players, collaborations, Licensing, Acquisition, Deal Value Trends Report 2020
Spinal Muscular Atrophy - Pipeline Insight, 2020,report provides comprehensive insights about 40+ companies and 40+ pipeline drugs in Spinal Muscular Atrophy Pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Geography Covered
- Global coverage
Key Players
- Kowa
- Scholar Rock
- Biogen
- Exicure
- AveXis
- Novartis Pharmaceuticals
- Catalyst Pharmaceuticals
- ReveraGen Biopharma
- Translate Bio
Spinal Muscular Atrophy: Overview
Spinal muscular atrophy (SMA) is a group of inherited disorders characterized by a loss of certain nerve cells in the spinal cord called motor neurons or anterior horn cells. Motor neurons receive the nerve impulses transmitted from the brain to the spinal cord (brainstem) and, in turn, transmit the impulses to the muscle via the peripheral nerves. The loss of motor neurons leads to progressive muscle weakness and muscle wasting (atrophy) in muscles closest to the trunk of the body (proximal muscles) such as the shoulders, hips and back. These muscles are necessary for crawling, walking, sitting up and head control. The more severe types of SMA can affect muscles involved in feeding, swallowing and breathing. SMA is divided into subtypes based on age of onset and maximum function achieved. SMA types 0, 1, 2, 3 and 4 are inherited as autosomal recessive genetic disorders and are associated with abnormalities (mutations) in the SMN1 and SMA2 genes which are located on chromosome 5.
Get Free Sample Copy of this Research Report at https://www.reportsnreports.com/contacts/requestsample.aspx?name=3715974
Symptoms
The primary symptom of chromosome 5-related (SMN-related) SMA is weakness of the voluntary muscles. The muscles most affected are those closest to the center of the body, such as those of the shoulders, hips, thighs, and upper back. The lower limbs seem to be affected more than the upper limbs, and deep tendon reflexes are decreased.
Special complications occur if the muscles used for breathing and swallowing are affected, resulting in abnormalities in these functions. If the muscles of the back weaken, spinal curvatures can develop.
Diagnosis
Spinal muscular atrophy (SMA) is sometimes difficult to diagnose, as symptoms can resemble other conditions or medical problems. Doctors usually diagnose SMA after a child has muscle weakness and decreased muscle tone.
If the clinician suspects SMA, they may use the following tests to diagnose the condition:
- Genetic blood tests, which can confirm the diagnosis of SMA
- An electromyography (EMG) test that measures the electrical activity of a muscle or a group of muscles (in some cases)
- A creatine kinase (CPK) test (to distinguish from other types of neuromuscular diseases, if necessary)
Get 20% Discount on this Research Report at https://www.reportsnreports.com/contacts/discount.aspx?name=3715974
Treatment
The management of children with spinal muscular atrophy starts with the diagnosis and classification into 1 of the 5 categories. Health issues specific to spinal muscular atrophy are as follows:
- Pulmonary management: Children with SMA1 can survive beyond 2 years of age when offered tracheostomy or noninvasive respiratory support.
- An intermittent positive-pressure breathing device (mechanical in-exsufflator) has proven effective.
- Nutrition: Bulbar dysfunction is universal in SMA1 patients. Early gastrostomy should be considered as part of the management of such patients. The bulbar dysfunction eventually becomes a serious problem for spinal muscular atrophy II patients and only very late in the course of disease for spinal muscular atrophy III patients.
- In 2017, Spinraza (nusinersen) was FDA-approved as the first drug to treat children and adults with SMA.
- In 2019, the FDA approved Zolgensma (onasemnogene abeparvovec-xioi) for the treatment of children less than two years of age with SMA.
- In 2020, the FDA approved Evrysdi (risdiplam) to treat patients two months of age and older with SMA. Evrysdi is the first orally administered drug approved for the treatment of SMA.
Spinal Muscular Atrophy Emerging Drugs Chapters
This segment of the Spinal Muscular Atrophy report encloses its detailed analysis of various drugs in different stages of clinical development, including phase II, I, preclinical and Discovery. It also helps to understand clinical trial details, expressive pharmacological action, agreements and collaborations, and the latest news and press releases.
Spinal Muscular Atrophy Emerging Drugs
- Sodium valproate: Kowa
Sodium valproate is being developed by Kowa for the treatment of Spinal muscular atrophy. It is currently in phase III stage of development.
- SRK-015: Scholar Rock
For More Details Inquire at https://www.reportsnreports.com/contacts/inquirybeforebuy.aspx?name=3715974
SRK-015, our most advanced product candidate, is a selective and local inhibitor of the activation of myostatin. Based on existing research on the mechanism of myostatin in muscle growth and strength, Scholar Rock believes the inhibition of the activation of myostatin with SRK-015 may promote a clinically beneficial increase in motor function. Scholar Rock is developing and investigating SRK-015 as a potential treatment to improve muscle strength and motor function in patients with Spinal Muscular Atrophy (SMA).it is currently in phase II stage of development.
- BIIB110: Biogen
BIIB110 is a hybrid activin II receptor (ACTIIR) ligand trap that sequesters both myostatin and activins while sparing the related ligand bone morphogen protein 9 (BMP9). This targeted mechanism of action may result in greater muscle mass, function and improved safety compared to other myostatin inhibition approaches. It is currently in phase I stage of development.
- Research programme: SNA based therapeutics: Exicure
SNA based therapeutics is being developed by Exicure for the treatment of Spinal muscular atrophy. It is currently in preclinical stage of development.
Further product details are provided in the report....
Spinal Muscular Atrophy: Therapeutic Assessment
This segment of the report provides insights about the different Spinal Muscular Atrophy drugs segregated based on following parameters that define the scope of the report, such as:
- Major Players in Spinal Muscular Atrophy
There are approx. 40+ key companies which are developing the therapies for Spinal Muscular Atrophy. The companies which have their Spinal Muscular Atrophy drug candidates in the most advanced stage, i.e. phase III include Kowa and others
- Phases
Report covers around 40+ products under different phases of clinical development like
- Late-stage products (Phase II and Phase II/III)
- Mid-stage products (Phase II and Phase II/III)
- Early-stage products (Phase I/II and Phase I) along with the details of
- Pre-clinical and Discovery stage candidates
- Discontinued & Inactive candidates
- Route of Administration
Spinal Muscular Atrophy pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
- Infusion
- Intradermal
- Intramuscular
- Intranasal
- Intravenous
- Oral
- Parenteral
- Subcutaneous
- Topical.
- Molecule Type
Direct Purchase of this Research Report at https://www.reportsnreports.com/purchase.aspx?name=3715974
Products have been categorized under various Molecule types such as
- Gene therapies
- Small molecule
- Vaccines
- Polymers
- Peptides
- Monoclonal antibodies
- Product Type
Drugs have been categorized under various product types like Mono, Combination and Mono/Combination.
Spinal Muscular Atrophy: Pipeline Development Activities
The report provides insights into different therapeutic candidates in phase II, I, preclinical and discovery stage. It also analyses Spinal Muscular Atrophy therapeutic drugs key players involved in developing key drugs.
Pipeline Development Activities
The report covers the detailed information of collaborations, acquisition and merger, licensing along with a thorough therapeutic assessment of emerging Spinal Muscular Atrophy drugs.
Report Highlights
- The companies and academics are working to assess challenges and seek opportunities that could influence Spinal Muscular Atrophy R&D. The therapies under development are focused on novel approaches to treat/improve Spinal Muscular Atrophy.
- In August 2020, Scholar Rock announced that the US Food and Drug Administration (FDA) has granted Rare Pediatric Disease (RPD) designation for SRK-015 for the treatment of Spinal Muscular Atrophy (SMA).
- In March 2018, Scholar Rock announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to its lead antibody product candidate, SRK-015, for the treatment of spinal muscular atrophy (SMA).
- In July 2018, Biogen announced the acquisition of the muscle enhancement programme from AliveGen, which includes candidate, BIIB 110 (previously known as ALG 801).
Spinal Muscular Atrophy Report Insights
- Spinal Muscular Atrophy Pipeline Analysis
- Therapeutic Assessment
- Unmet Needs
- Impact of Drugs
Spinal Muscular Atrophy Report Assessment
- Pipeline Product Profiles
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Products
- Sodium valproate
- SRK-015
- BIIB110
- Leadiant Biosciences
- Vamorolone
- Research programme: SNA based therapeutics
- Amifampridine Phosphate
- Nusinersen
- Onasemnogene
- Branaplam
- Valproic Acid
- Research programme: mRNA therapeutics
Spinal Muscular Atrophy Understanding
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Spinal Muscular Atrophy drugs?
- How many Spinal Muscular Atrophy drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Spinal Muscular Atrophy?
- What are the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Spinal Muscular Atrophy therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Spinal Muscular Atrophy and their status?
- What are the key designations that have been granted to the emerging drugs?
ReportsnReports.com is your single source for all market research needs. Our database includes 500,000+ market research reports from over 95 leading global publishers & in-depth market research studies of over 5000 micro markets.
+ 1 888 391 5441
sales@reportsandreports.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Spinal Muscular Atrophy Pipeline Market Global Insight, Key Players, collaborations, Licensing, Acquisition, Deal Value Trends Report 2020 here
News-ID: 2146250 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
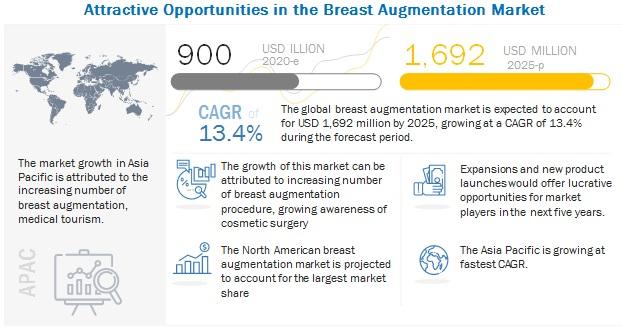
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for Spinal
Evolving Market Trends In The Spinal Fusion Devices Industry: Advancements In Sp …
The Spinal Fusion Devices Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories]._x000D_
_x000D_
What Is the Expected Spinal Fusion Devices Market Size During the Forecast Period?_x000D_
Over the past few years, there has been a significant growth in the spinal fusion devices market. The market is projected…
Key Influencer in the Spinal Fusion Devices Market 2025: Rising Spinal Disorder …
What Is the Forecasted Market Size and Growth Rate for the Spinal Fusion Devices Market?
The market for spinal fusion devices has experienced significant growth in the past couple of years. Expectations are that it will rise from estimates of $7.85 billion in 2024 to approximately $8.31 billion in 2025, giving us a compound annual growth rate (CAGR) of 5.8%. Factors contributing to this notable expansion during the historical period can…
Disposable Spinal Instrument Market: Revolutionizing Spinal Surgery with Hygiene …
Introduction
The medical device market has witnessed a surge in demand for disposable instruments, especially in areas where hygiene, precision, and efficiency are paramount. One such sector experiencing rapid growth is the disposable spinal instrument market. Spinal surgeries, being intricate and requiring high precision, have traditionally relied on reusable instruments. However, growing concerns about hygiene, infection control, and surgical efficiency have accelerated the shift toward disposable spinal instruments. These devices not…
Endoscopic Spinal Surgery Market: Minimally Invasive Solutions for Spinal Disord …
Global Endoscopic Spinal Surgery Market size was valued at US$ 667 Mn. in 2022 and the total revenue is expected to grow at a CAGR of 7.9% through 2022 to 2029, reaching nearly US$ 1137 Mn.
Endoscopic Spinal Surgery Market Overview:
Maximize Market Research is a research firm that has published a detailed analysis of the "Endoscopic Spinal Surgery Market". MMR in-depth market assessments in research reports consider significant technological advancements in…
Spinal Resilience: Acute Spinal Cord Injury Drug Pipeline Landscape (2023)
Market Outlook:
Charting Progress: Acute Spinal Cord Injury Drug Pipeline Landscape (2023)
The Acute Spinal Cord Injury Drug Pipeline Landscape sets its sights on a decade of advancements poised to redefine the trajectory of spinal resilience. This comprehensive outlook explores the unfolding strategies, innovations, and breakthroughs that promise to revolutionize the landscape of acute spinal cord injury treatment.
Market Drivers:
Neuroregeneration on the Horizon: At the forefront of the Acute Spinal Cord Injury Drug…
Spinal Laminoplasty Market - Revolutionizing spinal care with minimally invasive …
Newark, New Castle, USA: The "Spinal Laminoplasty Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Spinal Laminoplasty Market: https://www.growthplusreports.com/report/spinal-laminoplasty-market/8601
This latest report researches the industry structure, sales, revenue,…