Press release
Austrianova files Drug Master File with FDA
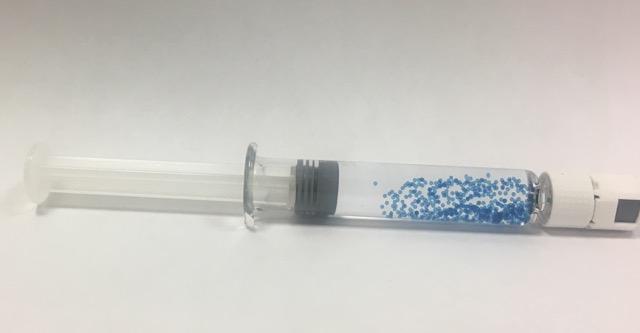
Syringe containing Cell-in-a-Box encapsulated cells. The capsules are stained blue for visualisation.
Austrianova’s CEO, Brian Salmons, said “The filing of this comprehensive DMF document is an important milestone event for Austrianova and represents a culmination of many years work on cell encapsulation based on this cell line. The DMF deposited with FDA describes confidential and detailed information about the facilities, processes, and materials used in the manufacturing, processing, packaging, and storing of Cell-in-a-Box® products based on HEK293 cells. The information contained in the DMF will be used to support PharmaCyte’s Investigational New Drug Application (IND) on CypCaps™ for the upcoming clinical trial for the treatment of pancreatic cancer.”
A DMF is a document that is submitted to a regulatory agency and contains complete information on an Active Pharmaceutical Ingredient and/or finished Drug Product. It comprises factual and complete information on a Drug Product's raw materials, process development and manufacture, analytical methods, stability, purity, packaging and its cGMP status. The DMF filing allows a company to protect its’ intellectual property from partners while complying with regulatory requirements for disclosure of processing details.
Austrianova, The Gemini, 41 Science Park Rd, Singapore 117610
Austrianova, (www.austrianova.com) part of the SG Austria Group, is a biotech company with a global footprint and headquarters in Singapore. Austrianova utilizes a novel and proprietary technology for the encapsulation of living mammalian (Cell-in-a-Box®) and bacterial (Bac-in-a-Box®) cells. Cell-in-a-Box® protects the encapsulated human or animal cells from rejection by the immune system and allows cells to be easily transported, stored and implanted at specific sites in patients. The technology, which has been proven safe and efficacious in clinical trials carried out in Europe, allows companies to develop a wide range of standard cells as one-for-all living pharmaceuticals. Bac-in-a-Box® is a similar protective device adapted for encapsulation of bacteria and yeast, including probiotics, and has both human food as well as animal feed applications due to its ability to extend storage under lyophilized conditions and to protect encapsulated bacteria against destruction by stomach acid. Austrianova now also offers GMP4Cells that includes competitively priced Master Cell Bank and Working Cell Bank production as well as “Fill and Finish” services for cell therapy products (such as stem cell therapies, biologics produced from cells e.g. vaccines, antibodies, recombinant proteins etc).
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Austrianova files Drug Master File with FDA here
News-ID: 2097898 • Views: …
More Releases from Austrianova
Austrianova reports successful metronomic treatment using Cell-in-a-Box® in an …
SINGAPORE, December 19, 2023 - Austrianova, a member of the SGAustria group of companies, and a leading provider of cell encapsulated products as well as other biological products and services, today announced a newly published study demonstrating that one administration of Cell-in-a-Box® encapsulated cells followed by multiple rounds (metronomic) of low dose ifosfamide controls tumor growth in a mouse model of pancreatic cancer.*
Chemotherapeutics such as ifosfamide and cyclophosphamide are normally…
Austrianova announces German subsidiary and new location in Singapore
SINGAPORE, November 1st, 2023 - SG Austria ("Austrianova"), a leading provider of cell biological products and services, today announced that it has established a German subsidiary company based in Munich and moved to a new location in Singapore.
The German subsidiary, Austrianova Germany, at "Am Klopferspitz" in Martinsried, the world renowned German biotech centre, will function as Austrianova's European base, expanding the company's activities and reach.
This year has been a…

Austrianova and ProAgni sign a licensing and manufacturing agreement for novel, …
Austrianova and ProAgni announced today the signing of a licensing and manufacturing agreement that foresees an intensification of joint activities between the two companies in the area of development and commercialization of shelf stable probiotic additives for cattle feed as well as for other farm animals.
Austrianova, a Singaporean biotech company, has developed its patented Bac-in-a-Box® technology specifically to efficiently protect bacteria from stomach acid as well as to allow…

Austrianova and San Raffaele University & Research Hospital announce the initiat …
The group of Dr. Matteo Bellone, group leader Cellular immunology Unit at San Raffaele University & Research Hospital, has shown that Prevotella melaninogenica, a commensal bacterium of the human gastrointestinal microbiota, can protect against the progression of relatively benign asymptomatic multiple myeloma to the aggressive form of the disease. Delivery of Prevotella melaninogenica in preclinical animal models prevents this progression but large doses of the bacteria are needed since they…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…