Press release
The DECISION project - European researchers seek to reduce the number of patients dying from cirrhosis
21 European institutions join forces to tackle end-stage liver disease and liver failure with a systems medicine approach- Despite a vast array of available interventions and medications, more than 1 million people die of chronic liver disease (cirrhosis) per year worldwide, when the disease progresses to decompensated cirrhosis and acute-on-chronic liver failure (ACLF), a state in which the dysfunctional liver induces failure of other organs.
- Following an acute decompensation of cirrhosis, 14% of the patients die of ACLF within 3 months. The reason why certain patients die and others survive is unknown, but huge differences between patients with regard to their individual genetics, medical history, precipitating events, clinical presentation and treatment response are suspected.
- These individual differences call for personalised treatments based on a precise understanding of underlying mechanisms. Systems medicine and high-throughput technology nowadays allow for highly efficient analysis, integration, and predictive modelling of clinical data to develop the best fitted, most personalised treatment for each patient.
- Over the next 5.5 years, the DECISION research consortium will analyse and integrate data from already existing clinical data and biological samples from 2,200 patients with cirrhosis at more than 8,600 time points to identify novel combinatorial therapies, validate them in animal models, and then test the most promising combinatorial therapy in a clinical trial.
- The overall aim of the DECISION project is to prevent ACLF and to significantly reduce the mortality rate amongst patients with decompensated cirrhosis. The project receives 6 million EUR funding from the European Commission.
Why is decompensated cirrhosis fatal?
End-stage chronic liver disease (cirrhosis) is a major cause of morbidity and mortality, and has a large socioeconomic impact because of high health care costs and the patients' inability to work or seek employment. Patients show symptoms, start suffering, and eventually die of chronic liver cirrhosis when the body essentially can't compensate the dysfunctional liver condition any longer. That's why it's called decompensated (as opposed to compensated) cirrhosis. Decompensated cirrhosis is defined by accumulation of fluid in the abdomen (ascites), impaired brain function (hepatic encephalopathy), and often also bleeding in the digestive tract (gastrointestinal haemorrhage). Eventually, it progresses to acute-on-chronic liver failure (ACLF) and death.
Despite a vast array of available treatments for decompensated cirrhosis, such as albumin, antibiotics and antiviral agents, anticoagulants, beta-blockers, diuretics, laxatives, proton-pump inhibitors, statins, steroids, and vasoconstrictors, 5% of the patients die by day 28, 14% within 90 days. Research suggests individual differences between patients with regard to genetics, precipitating events and treatment response as the main culprit for the high mortality rate. This heterogeneity calls for better patient stratification and treatment personalization based on underlying biological mechanisms.
How will patients with cirrhosis benefit from DECISION?
After identifying the most promising novel combinatorial treatment approaches via data analysis and predictive mathematical modelling in silico, DECISION researchers will first refine and optimise these therapies in animal models. Subsequently, the best combinatorial therapy will be tested in patients with cirrhosis in a phase II clinical trial. In addition, the DECISION consortium will develop two novel tests aiding hepatologists in everyday clinical decision making: one to reliably predict the therapeutic outcome in patients with decompensated cirrhosis when treated with standard treatment (prognostic test), the other one to successfully identify those patients who will respond best to the novel combinatorial therapy (test for predicted response). Thus, the patients participating in these clinical studies may directly benefit from DECISION research, while future patients with cirrhosis will hopefully benefit from novel combinatorial therapies and improved clinical guidelines derived from the project's findings and results.
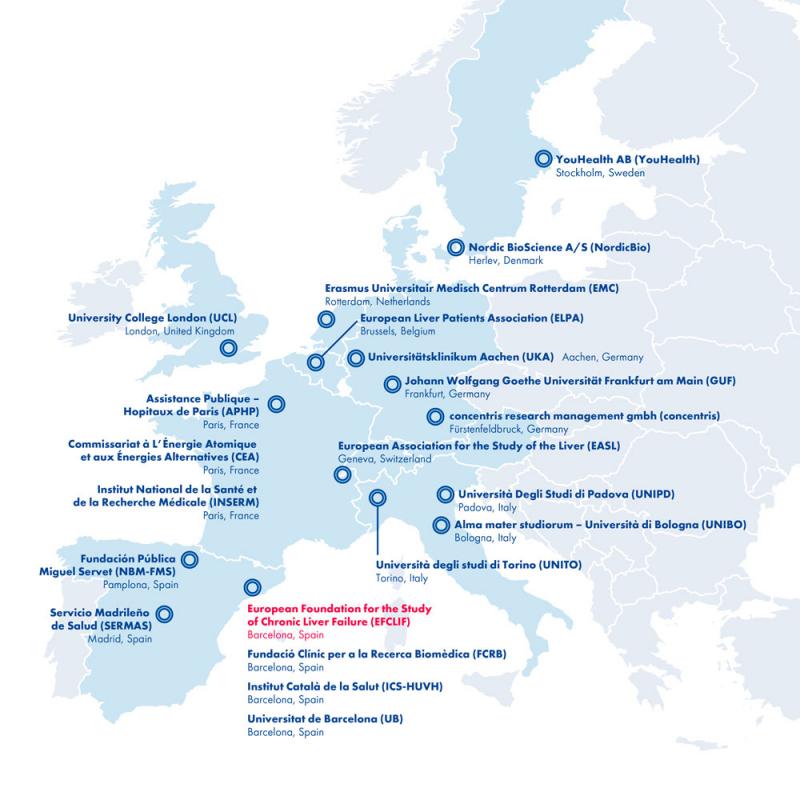
Who are the partners of the DECISION research consortium?
Professor Pierre-Emmanuel Rautou (MD, PhD), member of the European Foundation for The Study of Chronic Liver Failure (EFCLIF), Barcelona, and professor for hepatology at the French University de Paris (APHP) and the French Institut national de la sante et de la recherche medicale (INSERM), spearheads and coordinates DECISION. The 21 institutions that collaborate in this multi-centre project are spread throughout Europe and include hepatologists, molecular biologists, systems medicine specialists and patients' association. The multidisciplinary team strives for high-impact dissemination of scientific results and improved clinical guidelines:
1. Alma Mater Studiorum - Universita di Bologna (UNIBO), Italy
2. Assistance Publique Hopitaux de Paris (APHP) and University of Paris, France
3. Commissariat a l'Energie Atomique et aux Energies Alternatives (CEA), France
4. concentris research management GmbH (concentris), Germany
5. Erasmus Universitair Medisch Centrum Rotterdam (EMC), Netherlands
6. European Association for the Study of the Liver (EASL), Switzerland
7. European Foundation for the Study of Chronic Liver Failure (EFCLIF), Spain
8. European Liver Patients Association (ELPA), Belgium
9. Fundacio Clinic per a la Recerca Biomedica (FCRB), Spain
10. Fundacion Publica Miguel Servet (NBM-FSM), Spain
11. Institut Catala de la Salut (ICS-HUVH), Spain
12. Institut national de la sante et de la recherche medicale (INSERM), France
13. Johann Wolfgang Goethe-Universitaet Frankfurt Am Main (GUF), Germany
14. Nordic Bioscience A/S (NordicBio), Denmark
15. Servicio Madrileno de Salud (SERMAS), Spain
16. Universita degli Studi di Padova (UNIPD), Italy
17. Universita degli Studi di Torino (UNITO), Italy
18. Universitaetsklinikum Aaachen (UKA), Germany
19. Universitat de Barcelona (UB), Spain
20. University College London (UCL), United Kingdom
21. YH YouHealth AB (YouHealth), Sweden
Fuerstenfeldbruck, Germany, 14 April 2020
https://decision-for-liver.eu/
https://twitter.com/Decision4Liver
https://www.linkedin.com/company/decision-project
Funding
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 847949. This press release reflects only the view of the author or authors (scientific coordinator and contact & translating personnel), and the European Commission is not responsible for any use that may be made of the information it contains. Reproduction is authorised provided the source is acknowledged.
Prof. Pierre-Emmanuel Rautou
DECISION Coordinator
pierre-emmanuel.rautou@inserm.fr
+ 33 (0) 1 40 87 52 83
Dr. Nina Donner
Dissemination
nina.donner@concentris.de
+49 (0) 8141 6252 8584
Dr. Mary Gazea
Project Manager
mary.gazea@concentris.de
+49 (0) 8141 6252 8578
concentris research management gmbh
Ludwigstr. 4
82256 Fuerstenfeldbruck
Germany
concentris research management carries out the non-scientific tasks of funded research projects and provides support and consultancy services for scientists and researchers at universities, in businesses and research institutes from the first project idea to the successful completion. concentris supports national and international consortia with knowledge and concentration in project management, financial management and in the organisation of meetings.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The DECISION project - European researchers seek to reduce the number of patients dying from cirrhosis here
News-ID: 2012264 • Views: …
More Releases for DECISION
Clinical Decision Support App Market Clinical Decision Support App Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Clinical Decision Support App Market Size, Share & Trends Analysis Report By Delivery Platform (Web-Based CDS Apps, Mobile-Based CDS Apps), By End User (Hospitals And Clinics, Ambulatory Care Centres, Long-Term Care Facilities), By Application (Diagnostic Support, Treatment Decision Support, Drug Interactions And Safety), By Region, And By Segment Forecasts, 2024-2031"
According to the latest research by InsightAce…
Decision Management Market
The Business Research Company has recently revised its global market reports, now incorporating the most current data for 2024 along with projections extending up to 2033.
Decision Management Global Market Report 2024 by The Business Research Company offers comprehensive market insights, empowering businesses with a competitive edge. It includes detailed estimates for numerous segments and sub-segments, providing valuable strategic guidance.
The Market Size Is Expected To Reach $14.00 Bn In 2028 At…
World Leading Companies involved in The Growing Management Decision Solutions …
The latest research study released by Worldwide Market Reports on "Management Decision Solutions Market 2023" holds tons of experience in offering comprehensive and accurate analysis of global as well as regional markets. The analysts and researchers authoring the report have provided a deeper competitive analysis of the Management Decision Solutions market along with exhaustive company profiling of leading market players. This research study of the Management Decision Solutions Market involved…
Decision making software Market Is Booming Worldwide : Information Builders, Dec …
Latest Study on Industrial Growth of Decision making software Market 2023-2028. A detailed study accumulated to offer Latest insights about acute features of the Decision making software market. The report contains different market predictions related to revenue size, production, CAGR, Consumption, gross margin, price, and other substantial factors. While emphasizing the key driving and restraining forces for this market, the report also offers a complete study of the future trends…
Management Decision Solutions Market Share, Size and Industry Growth - ACTICO Gm …
The Management Decision Solutions Industry report provides general consumption trends, sales techniques, and top Management Decision Solutions industry nations. The research examines providers in the global market segmentation, competition, and the macroeconomic climate. A complete Management Decision Solutions analysis takes into account population and business cycles, as well as market-specific microeconomic consequences. The global market research also includes a specific competition landscape section to help you better understand the Management…
Global Decision Making Software Market, Global Decision Making Software Industry …
The Decision-making Software market is expected to grow from USD X.X million in 2020 to USD X.X million by 2026, at a CAGR of X.X% during the forecast period. The Global Decision-Making Software Market report is a comprehensive research that focuses on the overall consumption structure, development trends, sales models and sales of top countries in the global Decision-making Software market. The report focuses on well-known providers in the global…