Press release
New Report Explores Hottest Trends in Biosimilar Drug Market 2020-2025: Global Key Vendors - Amgen, Eli Lilly, Novartis, CP Guojian Pharma, Biotech Pharma
Global Biosimilar Drug Market Synopsis:The exclusive research report on the Global Biosimilar Drug Market 2020-2025 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global Biosimilar Drug Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Major key factors driving the growth of the Global Biosimilar Drug Market are rapid growth in incidences of chronic diseases globally, coupled with the cost-effectiveness of biosimilars. In addition, the development of biosimilars for new indications and the patent expiry of biologic drugs are expected to offer significant growth opportunities to the Biosimilar Drug Market players in the coming years.
Available Exclusive Sample Copy of this Report @ https://www.businessindustryreports.com/sample-request/254178 .
Significant points in table of contents: Market Definition, Market Overview, Business Introduction, Segmentation (Region Level), Segmentation (Type Level), Segmentation (Industry Level), Segmentation (Channel Level), Market Forecast Year, Segmentation Type, Segmentation Industry, Market Cost Analysis, and Conclusion. The Biosimilar Drug Market is segmented based on Product, source, application and Regions. On the basis of product, the market is sub-segmented.
Regionally, North America and Europe holds major share in Global Biosimilar Drug Market. Moreover, Asia-Pacific is expected to record higher growth rate in Biosimilar Drug market during the forecast year.
Top Major Key Players in the Global Biosimilar Drug Market:
1 Amgen
2 Eli Lilly
3 Novartis
4 CP Guojian Pharma
5 Biotech Pharma and More............
Purchase this report online with 90 Pages, List of Tables & Figures and in-depth Table of Contents on "Global Biosimilar Drug Market Report 2020" @ https://www.businessindustryreports.com/buy-now/254178/single .
Product Type Segmentation
1 Injection
2 Tablets
3 Other Types
Industry Segmentation
1 Ankylosing Spondylitis
2 Tumor
3 Rheumatoid Arthrtis
4 Cardiovascular
Top Medical Industry News:
Novartis (March 19, 2020) - Novartis receives approval from Japanese Ministry of Health, Labour and Welfare for Zolgensma the only gene therapy for patients with spinal muscular atrophy (SMA) - Novartis Pharma K.K. ("Novartis Pharma") today announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved Zolgensma (onasemnogene abeparvovec) for the treatment of spinal muscular atrophy (SMA) in patients under the age of two, including those who are pre-symptomatic at diagnosis. Patients must be negative for elevated anti-AAV9 antibodies. A rare, genetic neuromuscular disease caused by a lack of a functional SMN1 gene, SMA results in the rapid and irreversible loss of motor neurons, affecting muscle functions, including breathing, swallowing and basic movement. Approximately 60% of all SMA is Type 1. Zolgensma is a one-time gene therapy designed to address the genetic root cause of the disease by replacing the function of the missing or nonworking SMN1 gene. Zolgensma is administered during a single intravenous (IV) infusion, delivering a new working copy of the SMN gene into a patient's cells, halting disease progression. Approximately 15-20 SMA patients in Japan are expected to be eligible for treatment each year. Reimbursement with MHLW is expected by the end of 1H20 and, pending agreement, Zolgensma will be available at that time.
"SMA is the leading genetic cause of infant death and, if left untreated in its most common form, Type 1, leads to death or the need for permanent ventilation by the age of two in more than 90% of cases," said Kazunari Tsunaba, president and representative director, Novartis Pharma. "A one-time dose of Zolgensma has the potential to make a truly transformative impact on this life-threatening disease. This is an important day for the children and families in Japan impacted by SMA, both today and in the future."
The most commonly observed side effects after treatment were elevated liver enzymes and vomiting. Acute serious liver injury and elevated aminotransferases can occur. Patients with pre-existing liver impairment may be at higher risk. Prior to infusion, physicians should assess liver function of all patients by clinical examination and laboratory testing. And, they should administer systemic corticosteroid to all patients before and after treatment, and then continue to monitor liver function for at least 3 months after infusion.
Grab Your Report at an Impressive Discount @ https://www.businessindustryreports.com/check-discount/254178 .
Major Points in Table of Contents:
Global Biosimilar Drug Market Report 2020
1 Biosimilar Drug Product Definition
2 Global Biosimilar Drug Market Manufacturer Share and Market Overview
2.1 Global Manufacturer Biosimilar Drug Shipments
2.2 Global Manufacturer Biosimilar Drug Business Revenue
2.3 Global Biosimilar Drug Market Overview
3 Manufacturer Biosimilar Drug Business Introduction
3.1 Amgen Biosimilar Drug Business Introduction
3.2 Eli Lilly Biosimilar Drug Business Introduction
3.3 Novartis Biosimilar Drug Business Introduction
3.4 CP Guojian Pharma Biosimilar Drug Business Introduction
3.5 Biotech Pharma Biosimilar Drug Business Introduction
About us
BusinessindustryReports.com is digital database of comprehensive market reports for global industries. As a market research company, we take pride in equipping our clients with insights and data that holds the power to truly make a difference to their business. Our mission is singular and well-defined - we want to help our clients envisage their business environment so that they are able to make informed, strategic and therefore successful decisions for themselves.
Media Contact
Business Industry Reports
Pune - India
sales@businessindustryreports.com
+19376349940
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release New Report Explores Hottest Trends in Biosimilar Drug Market 2020-2025: Global Key Vendors - Amgen, Eli Lilly, Novartis, CP Guojian Pharma, Biotech Pharma here
News-ID: 2002861 • Views: …
More Releases from Business Industry Reports
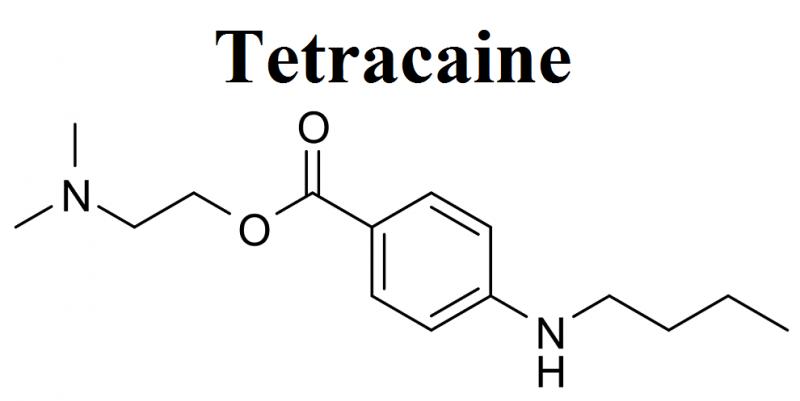
Tetracaine Market Exhibits a Lucrative Growth Potential during 2021-2025 | Endo …
BusinessIndustryReports has recently broadcasted a new study to its broad research portfolio, which is titled as “Global Tetracaine Market” Research Report 2021 provides an in-depth analysis of the Tetracaine with the forecast of market size and growth. The analysis includes addressable market, market by volume, market share by business type and by segment (external and in-house). The research study examines the Tetracaine on the basis of a number of criteria,…

Cyber Warfare Market Evenly Poised To Reach A Market Value of US$ By Share, Size …
Overview of Global Cyber Warfare Market:
This report provides in-depth study of “Global Cyber Warfare Market 2021” using SWOT analysis i.e. Strength, Weakness, Opportunities, and Threat to the organization. The Cyber Warfare Market report also provides an in-depth survey of key players in the market organization.
According to the market research study, the Cyber Warfare is virtual conflict between state, organization, or country by the use of computer technology to disrupt activities…
Covid-19 Impact on Cranio Maxillofacial Implant Market 2021-2025: Business Growt …
Overview of Global Cranio Maxillofacial Implant Market:
This report provides in-depth study of “Global Cranio Maxillofacial Implant Market 2021” using SWOT analysis i.e. Strength, Weakness, Opportunities, and Threat to the organization. The Cranio Maxillofacial Implant Market report also provides an in-depth survey of key players in the market organization.
According to the market research study, Craniomaxillofacial Implants are medical implants used in surgeries of maxillofacial region such as, head, face, neck, oral,…

Beginning of the bloom: The Rise of the Biohacking Market 2021-2025 | Global Key …
Global Biohacking Market Synopsis:
The report covers a forecast and an analysis of the Biohacking Market on a global and regional level. The study provides historical data for 2015, 2016, 2017 and 2018 along with a forecast from 2020 to 2025 based on revenue (USD Million) and volume (Kilotons). The study includes drivers and restraints of the Biohacking Market along with the impact they have on the demand over the forecast…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…