Press release
ECLINICAL SOLUTIONS MARKET FUTURE OUTLOOK AND BY TOP KEY PLAYERS ANALYSIS PAREXEL INTERNATIONAL CORPORATION, ORACLE, MEDIDATA SOLUTIONS, INC., VEEVA SYSTEMS
eClinical Solutions Market report is a synopsis about how is the market status right now and how will it be in the forecast years for industry. Furthermore, Global eClinical Solutions Market report can be explored in terms of breakdown of data by manufacturers, region, type and application, market status, market share, growth rate, future trends, market drivers, opportunities and challenges, emerging trends, risks and entry barriers, sales channels, and distributors. To understand the competitive landscape in the market, an analysis of Porter's five forces model for the market has also been included. Global eClinical Solutions Market report puts light on the types of customers, product-buyer insights, market changes over last few years, reactions of various geographic regions, new developments in the market, actions of other corporate players and more.Global eClinical Solutions Market is projected to register a healthy CAGR of 13.4% in the forecast period of 2019 to 2026.
Get Sample Report + All Related Graphs & Charts @
https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-eclinical-solutions-market
eClinical solutions are comprised of electronic health record, electronic consent forms, integrating eTechnologies, electronic data capture and clinical data management systems. eClinical solutions are helping researchers in end-to-end clinical research process providing solution, through proper management of lengthy clinical research process. Advancement in technology and government initiatives raises the market of eClinical solutions. It helps the clinical research organizations with regulatory document management, team collaboration and management of supply chains, site performance management and reporting which is increasing the demand of eClinical solutions in the market in the forecast period.
Major Players: Global eClinical Solutions Market
Some of the major players operating in global eClinical solutions market are Parexel International Corporation, Oracle, Medidata Solutions, Inc., Veeva Systems, ERT Clinical, eClinical Solutions LLC, IBM Corporation, eClinicalWorks, Bioclinica, ArisGlobal, Advarra, Anju Software, Inc., Bio-Optronics, Inc., DATATRAK Int., ICON plc, MasterControl, Inc., MaxisIT, OmniComm Systems, Inc., RESONANCE HEALTH, The Realtime Group, Signant Health, Xybion Corporation among others.
Global eClinical Solutions Market By Product {Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS), Randomization and Trial Supply Management (RTSM), Clinical Trial Management Systems (CTMS), Electronic Clinical Outcome Assessment (ECOA), Electronic Trial Master File Systems, Safety Solutions, Regulatory Information Management Solutions, Clinical Data Integration Platforms, Other eClinical Solutions}, Deployment {Web-Hosted (On-Demand) Solutions, Cloud-Based (SaaS) Solutions, Licensed Enterprise (On-Premise) Solutions}, Clinical Trial Phase (Phase I, Phase II, Phase III, Phase IV), End User {Hospital/Healthcare Provider, Contract Research Organizations (CROS), Pharma & Biotech Organizations, Medical Device Manufacturers, Consulting Service Companies, Academic Institutes}, Geography (North America, Europe, Asia-Pacific, South America, Middle East and Africa) - Industry Trends and Forecast to 2026
Drivers: Global eClinical Solutions Market
Increasing adoption of clinical trials
Growing number of contract research organization
Restraints:
Dearth of trained professionals
High implementation cost
Opportunity:
Strategic initiative by the market players
Challenge:
Stringent regulations
Market Trends:
The global eClinical solutions market is segmented into four notable segments which are product, deployment, clinical trial phase and end user.
On the basis of product, the market is segmented into electronic data capture (EDC) and clinical data management systems (CDMS), randomization and trial supply management (RTSM), clinical trial management systems (CTMS), electronic clinical outcome assessment (ECOA), electronic trial master file systems, safety solutions, regulatory information management solutions, clinical data integration platforms and other eClinical solutions. In 2019, electronic data capture (EDC) and clinical data management systems (CDMS) segment is expected to dominate the market with largest market share because these are mostly used after the clinical trials are done. This is due to high number of conducting trails, high demand for eDiaries and clinical studies. Due to these EDC and CDMS product segment is growing and the number of trials are captured in the solution and kept for further examinations.
On the basis of deployment, the market is segmented web-hosted (on-demand) solutions, cloud-based (SaaS) solutions and licensed enterprise (on-premise) solutions.
On the basis clinical trial phase, the market is segmented into Phase I, Phase II, Phase III and Phase IV. In 2019, Phase III segment is expected to dominate the market with largest market share as eClinical solutions phases are most crucial part and phase III accounts for the major portion as the clinical trials are mostly tested in the phase III and once they are cleared for this phase they are headed to further phase and then FDA approvals.
On the basis of end user, the market is segmented into hospital/healthcare provider, contract research organizations (CROS), pharma & biotech organizations, medical device manufacturers, consulting service companies, academic institutes.
Grab Your Report at an Impressive 30% Discount! Please click Here@ https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-eclinical-solutions-market
Segmentation: Global eClinical Solutions Market
The global eClinical solutions market is segmented into four notable segments which are product, deployment, clinical trial phase and end user.
On the basis of product, the market is segmented into electronic data capture (EDC) and clinical data management systems (CDMS), randomization and trial supply management (RTSM), clinical trial management systems (CTMS), electronic clinical outcome assessment (ECOA), electronic trial master file systems, safety solutions, regulatory information management solutions, clinical data integration platforms and other eClinical solutions.
In September 2019, Larry Ellison Chairman and CTO of announced their combined technology named as Oracle Generation 2 Cloud Infrastructure. This amalgamation of technology will improve database, analytics, Security, integration, and extensibility capabilities. With, this technology Oracle will able to stabilize its position in cloud application market.
On the basis of deployment, the market is segmented web-hosted (on-demand) solutions, cloud-based (SaaS) solutions and licensed enterprise (on-premise) solutions.
In September 2019, Oracle expanded its platform for utility of customer innovation; this expansion will be a best chance for Oracle, to stabilize itself in front of the customers. With this expansion oracle will able to expand its market.
On the basis clinical trial phase, the market is segmented into Phase I, Phase II, Phase III and Phase IV.
In June 2019, DASSAULT SYST»MES acquired the Medidata cloud based services. This acquisition will bring a major technological change in the field of bioinformatics and precision medicine. This acquisition will broaden the product portfolio of both the organization.
On the basis of end user, the market is segmented into hospital/healthcare provider, contract research organizations (CROS), pharma & biotech organizations, medical device manufacturers, consulting service companies, academic institutes.
In September 2019, Parexel International Corporation expanded phase 1 research in Greater China to improve the support level for customers conducting phase-1 research. With this expansion the company will expand its product portfolio in the market.
Product Launch
In March 2019, ERT Clinical launches India's first purpose built, product named as SpiroSphere spirometer, which will be use in clinical trial, it will captures research-grade clinical data and will provide better focus on both the data quality and patient. This will lead to clinical trial sponsors to confidently evaluate the efficacy and safety. This will lead to develop the market of ERT Clinical.
In March 2019, ArisGlobal, a leading provider of life sciences software, announced the launch of LifeSphere Publishing and LifeSphere EasyDocs, which will be providing prior information about validation and submissions management system to easily plan, publish, Compile and validate regulatory submissions, which will give company a better CTD reporting method.
In February 2019, Anju Software, the leading healthcare technology and analytics platform provider, launched the web-based coding system. This system will give easy way to handle the clinical trial. This software can be used while capturing the data of clinical trial. By this Anju software can add the value to the product, and will expand their market.
Note: If you have any special requirements, please let us know and we will offer you the report as you want.
Contact:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: Corporatesales@databridgemarketresearch.com
About Data Bridge Market Research:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release ECLINICAL SOLUTIONS MARKET FUTURE OUTLOOK AND BY TOP KEY PLAYERS ANALYSIS PAREXEL INTERNATIONAL CORPORATION, ORACLE, MEDIDATA SOLUTIONS, INC., VEEVA SYSTEMS here
News-ID: 1984511 • Views: ‚Ķ
More Releases from Data Bridge Market Research

Scented Candle Market Shows Strong Growth Driven by Wellness and Home DeŐĀcor Tr ‚Ķ
The global scented candle market is on track for significant expansion, increasing from an estimated USD 3.60 billion in 2024 to USD 6.00 billion by 2032, registering a strong CAGR of 6.60%. Rising consumer interest in home ambiance, wellness, and premium lifestyle products continues to drive market demand.
Get More Detail: https://www.databridgemarketresearch.com/reports/global-scented-candle-market
Market Growth Drivers
The scented candle market has evolved beyond being just a decorative item. Key growth factors include:
Home Fragrance &…
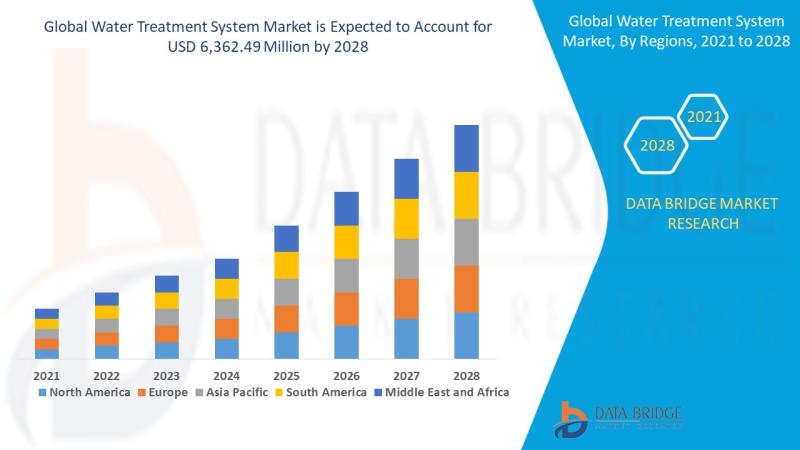
Water Treatment System Market: Sustaining the Future of Clean Water
Introduction
Understanding Water Treatment Systems
Water treatment systems are designed to purify and disinfect water for various uses-drinking, industrial processes, irrigation, and wastewater reuse. These systems eliminate contaminants such as bacteria, viruses, heavy metals, chemicals, and particulates, making water safe and sustainable for consumption and use.
Importance in Global Sustainability
Clean water is essential to life and industrial progress. With growing water demand and pollution, water treatment systems are now critical infrastructure across the…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360¬į view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…