Press release
The contract regulatory affairs-management services market for medical devices is estimated to be worth USD 820 million by 2030, growing at a CAGR of 6.9%, claims Roots Analysis
In order to reduce risk of device recalls and expedite time to market, medical device developers rely on contract service providers for regulatory submissions and navigating various challenging aspects of the product review and approval processRoots Analysis has announced the addition of "Contract Regulatory Affairs-Management Market for Medical Devices, 2019-2030" report to its list of offerings.
More than 130 medical devices were approved by the FDA since January 2018, while several are currently being evaluated across more than 9,500 (active) clinical trials, worldwide. Even though technical innovation has enabled the development of a variety of versatile medical devices, product approval, given stringent regulatory standards, is still a concern.
To order this 550+ page report, which features 245+ figures and 250+ tables, please visit this link
Key Market Insights
Over 400 CROs claim to offer regulatory affairs-management services for medical devices
Of these, over 40% players provide the aforementioned services for all device classes. Further, the market is characterized by the presence of companies of all sizes; it is also worth noting that very small and small companies are solely comprised of more than 50% of the competitive landscape.
About 30% of companies have elaborate service portfolios, offering end-to-end solutions
Most players (78%) offer assistance in regulatory document submissions; other popular services (in terms of number of companies offering them) include vigilance and medical device reporting (74%), followed by technical dossier set-up (72%), product registration and clinical trial applications (67%), risk management-related services (65%), and regulatory writing and publishing (59%).
60% CROs offer support for launching medical devices in developed regions
Majority of the service providers are experienced in enabling their sponsors to have their products launched in the US and are capable of efficiently navigating the regulatory framework established by the US FDA. Regarding developing regions, many CROs presently claim to be familiar with the regulatory environments in China (18%), Australia (16%) and India (13%).
Quality and reliability are considered to be important parameters while selecting a CRO
Nearly 75% big pharma players stated that the aforementioned parameters were crucial for evaluating the performance of a potential contract services partner. Other important performance metrics that emerged in this assessment include flexibility, financial stability, and cost and time management.
North America and Europe are anticipated to capture over 65% the market share by 2030
In addition, the market in the Asia-Pacific is projected to grow at a relatively faster rate (~24%). Further, in terms of device class, class II medical devices currently represent the highest share (48%); this trend is unlikely to change in the foreseen future.
To request a sample copy / brochure of this report, please visit this link
Key Questions Answered
Who are the leading CROs offering regulatory affairs-management services for medical devices?
What are differences in regulatory guidelines for medical device approval, across various geographies?
What are the key performance indicators used by sponsors to evaluate potential service providers?
What are the popular outsourcing models used by medical device companies for regulatory affairs-management purposes?
What are the key challenges faced by medical device developers / manufacturers in terms of regulations related to medical device approvals?
How is the current and future market opportunity likely to be distributed across key market segments?
The USD 820 million (by 2030) financial opportunity within the contract regulatory affairs-management services market for medical devices has been analyzed across the following segments:
Medical Device Class
Class I
Class II
Class III
Therapeutic Area
Cardiovascular Disorders
CNS Disorders
Metabolic Disorders
Oncological Disorders
Ophthalmological Disease
Orthopedic Disorders
Pain Disorders
Respiratory Disorders
Others
Type of Regulatory Affairs Service
Pharmacies GAP-Analysis
Pharmacies Legal Representation
Pharmacies Notified Body Selection
Product Labelling-related Services
Product Registration and Clinical Trial Applications
Regulatory Document Submissions
Regulatory Writing and Publishing
Risk Management-related Services
Technical Dossier Set-up
Vigilance & Medical Device Report
Key Geographical Regions
North America
Europe
Asia-Pacific and Rest of the World
The report features inputs from eminent industry stakeholders, according to whom, presently, regulatory affairs management, clinical trial site selection, trial monitoring and project management are the activities outsourced by most of the sponsor companies. Further, the requirement for highly experienced personnel for conducting such activities has compelled developers to outsource their regulatory affairs management operations, since most of the players are inherently inexperienced in navigating through the complex regulatory frameworks. The report includes detailed transcripts of discussions held with the following experts:
Troy McCall (Chief Operating Officer, CROMSOURCE)
Antal Solyom (Director, Medical Device Unit, HungaroTrial)
C Omprakash (Technical Director and Partner, Vyomus Consulting)
Nazish Urooj (Senior Manager, Medical & Clinical Operations, Metrics Research)
Christian Wolflehner (General Manager, CW Research & Management)
Alexa Foltin-Mertgen (Business Development Manager, AtoZ-CRO)
Tania Persson (Business Development Manager, A+ Science)
The research covers detailed profiles, featuring an overview of the company, its financial information (if available), regulatory affairs-based service portfolio, recent developments and an informed future outlook.
CTI Clinical Trial and Consulting Services
CROMSOURCE
ICON
Intertek
Medpace
MIC Medical
North American Science Associates (NAMSA)
Parexel
PharmaLex
Premier Research
Société Générale de Surveillance (SGS)
Underwriters Laboratory (UL)
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/med-dev-regulatory/282.html
or email sales@rootsanalysis.com
You may also be interested in the following titles:
Viral Vectors, Non-Viral Vectors and Gene Therapy Manufacturing Market (3rd Edition), 2019-2030
Medical Device Labels Manufacturing Market, 2019-2030
Medical Device Contract Manufacturing Market, 2019-2030
Contact:
Gaurav Chaudhary
+1 (415) 800 3415
Gaurav.Chaudhary@rootsanalysis.com
A 430, Bestech Business Towers,, Sector 66, Sahibzada Ajit Singh Nagar, Punjab 160066
Roots Analysis offers market research reports highlighting insightful opinions within the pharma, biotech and medical devices industry. With over 500 clients spread across the small pharma, large pharma, VC firms and academic institutes, our intellectual capital encompasses very niche / emerging market segments.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The contract regulatory affairs-management services market for medical devices is estimated to be worth USD 820 million by 2030, growing at a CAGR of 6.9%, claims Roots Analysis here
News-ID: 1970336 • Views: …
More Releases from Roots Analysis
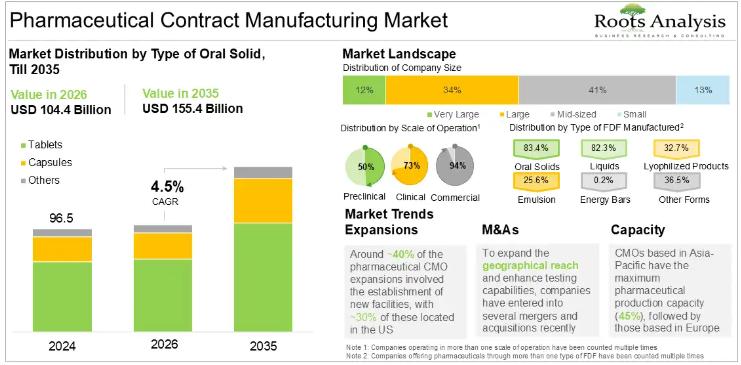
Pharmaceutical Contract Manufacturing Market CAGR To Reach 4.5% between 2025 and …
According to our latest market report "Pharmaceutical Contract Manufacturing Market by Type of Product Manufactured, Type of API, API Potency, Type of FDF, Dosage Form, Type of Oral Solid, Type of Packaging Offered, Scale of Operation, End User, Geographical Regions and Key Players: Industry Trends and Global Forecasts, till 2035", the pharmaceutical contract manufacturing market is estimated to be USD 100.3 billion in 2025. It is expected to reach USD…
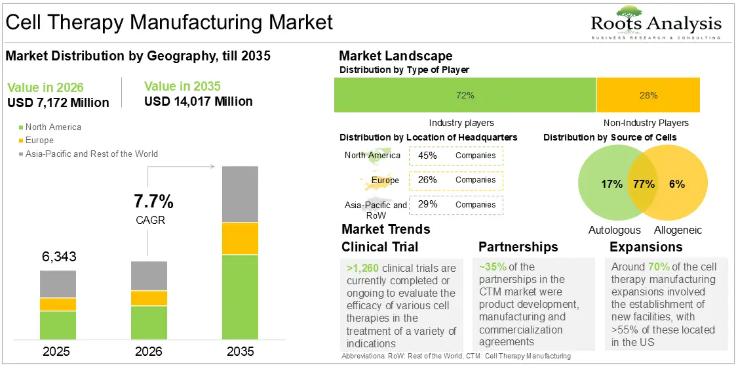
Cell Therapy Manufacturing Market CAGR To Exceed 8.25% by 2035, Due to the Growi …
According to our latest market report "Cell Therapy Manufacturing Market by Type of Cell Therapy, Source of Cells, Scale of Operation, Type of Manufacturer and Key Geographical Regions: Industry Trends and Global Forecasts, 2023-2035", the global cell therapy manufacturing market size is projected to reach USD 14,017 million by 2035 from USD 6,343 million in 2025, growing at a CAGR of 8.25% in the forecast period 2025-2035.
To request quote…
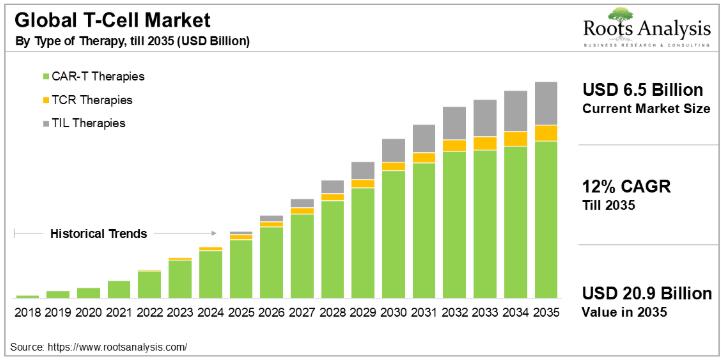
T-Cell Therapy Market Size to Hit USD 20.9 billion by 2035| Exclusive Report by …
Cancer is one of the leading causes of mortality across the world. As per the International Agency for Research on Cancer (IARC), by 2040, there are likely to be 27.5 million new cases and 16.3 million deaths related to cancer, annually. Although cancer therapeutics continue to be one of the most active areas, in terms of drug development, there is still a significant unmet need in this domain. In fact,…
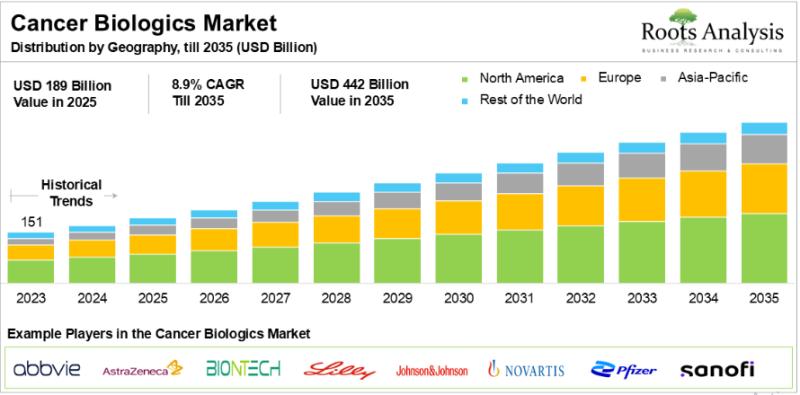
Cancer Biologics Market: Unmet Need and Treatment Guidelines
Owing to the increasing mortality rates and growing need for novel modalities to treat oncological disorders, several researchers and industry stakeholders have shifted their focus on the development of safe and effective biologic therapies. Cancer biologics are the class of therapeutic agents, which primarily modulate immune responses or directly inhibits oncogenic pathways in malignancies. These therapies, such as monoclonal antibodies, specifically target tumor-activating genes, facilitate antibody-dependent cellular cytotoxicity and complement…
More Releases for Medical
ECG Analysis System Market Research Report 2022 - GE Medical, Medical Econet, Gr …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global ECG Analysis System. On the basis of historic growth analysis and current scenario of ECG Analysis System place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
ECG Analysis System Market Outlook: 2020 The Year On A Positive Note | GE Medica …
DataIntelo report titled Global ECG Analysis System Market provides detailed information and overview about the key influential factors required to make well informed business decision. This is a latest report, covering the current COVID-19 impact on the market. The pandemic of Coronavirus (COVID-19) has affected every aspect of life globally. This has brought along several changes in market conditions. The rapidly changing market scenario and initial and future assessment of…
Medical Tracheostomy Tube Market Overall Study Report 2020-2027 | Players Medtro …
The latest report added by Stratagem Market Insights gives deep insights into the drivers and restraints in the Worldwide Medical Tracheostomy Tube Market. The research report "Global Medical Tracheostomy Tube Market Size and Growth Forecast to 2027" provide a comprehensive take on the overall market. Analysts have carefully evaluated the milestones achieved by the global Medical Tracheostomy Tube market and the current trends that are likely to shape its future.…
Global Embolization Particle Market 2019 - Sirtex Medical, Merit Medical, Cook M …
The global "Embolization Particle Market" report delivers a comprehensive and systematic framework of the Embolization Particle market at a global level that includes all the key aspects related to it. The data is collected from different sources allied to the global Embolization Particle market and the research team meticulously analyze the gathered data with the help of various analytical tools and present their opinion based on analysis and calculations. The…
Medical Casting & Splinting Market 2019: Top Key players are 3M, DJO Global, BSN …
Medical Casting & Splinting Market 2019 Report analyses the industry status, size, share, trends, growth opportunity, competition landscape and forecast to 2025. This report also provides data on patterns, improvements, target business sectors, limits and advancements. Furthermore, this research report categorizes the market by companies, region, type and end-use industry.
Get Sample Copy of this Report@ https://www.researchreportsworld.com/enquiry/request-sample/13718924
Global Medical Casting & Splinting market 2019 research provides a basic overview of…
Global Oxygen Pressure Regulator Market 2017 : Precision Medical, Smiths Medical …
Oxygen Pressure Regulator
A market study based on the " Oxygen Pressure Regulator Market " across the globe, recently added to the repository of Market Research, is titled ‘Global Oxygen Pressure Regulator Market 2017’. The research report analyses the historical as well as present performance of the worldwide Oxygen Pressure Regulator industry, and makes predictions on the future status of Oxygen Pressure Regulator market on the basis of this analysis.
Get Free…