Press release
Pharmacovigilance and Drug Safety Software Market Will Grow During Forecasts Period 2019-2026
Pharmacovigilance and Drug Safety Software MarketThe Global Pharmacovigilance and Drug Safety Software Market research report provides a unique methodology for evaluating market insights, highlighting opportunities and supporting strategic and tactical decision-making. This report recognizes the requirement of a rapidly-evolving and competitive environment, up-to-date marketing information is essential to monitor performance and make critical decisions for growth and profitability. It provides information on the latest trends and developments and focuses on innovations with emerging new technologies, and on the changing structure of the Pharmacovigilance and Drug Safety Software market.
To obtain a Sample copy of this report, click here @ https://straitsresearch.com/report/Pharmacovigilance-and-Drug-Safety-Software-Market/request-sample
The research study evaluates the overall size of the Global Pharmacovigilance and Drug Safety Software Market, by making use of a bottom-up approach, wherein data for different industry verticals, and end-user industries and its applications across various product types have been recorded and predicted during the forecast period. These segments and sub-segments have been documented from the industry specialists and professionals, as well as company representatives, and are outwardly validated by analyzing previous years’ data of these segments and sub-segments for getting an accurate and complete market 2019-26 size. The market also offers different auxiliary sources, such as company insights, financial reports, press releases, company annual reports, and investor presentations.
Some of the key players in the pharmacovigilance and drug safety software market Sarjan Systems Pvt Ltd, Sparta Systems INC, United BioSource Corporation, Oracle Corporation, Online Business Application INC, and EXTEBO Gmbh.
The research report tracks the major market events including product launches, technological developments, mergers & acquisitions, and the innovative business strategies opted by key market players. Along with strategically analyzing the key micro trends in the global markets, the report also focuses on industry-specific drivers, restraints, opportunities, and challenges in the Pharmacovigilance and Drug Safety Software market.
The Pharmacovigilance and Drug Safety Software market has been witnessing a considerable change in its size and value. The report presents an in-depth analysis of the various segments and sub-segments of the market, including the product types, upcoming technologies, application developments, industry verticals, and regions that are expected to dominate the market during the forecast period 2019-26. The report also presents a detailed overview of the market, which comprises of the key definitions and the key trends witnessed in the previous years.
The report has been gathered by making use of both primary and secondary research methodologies. A list of the major industry participants has also been mentioned in this research study, after which a primary research study has been undertaken with the enlisted key players. The primary research methodology also studied the service offerings, M&A, distribution and channels, and all major partnerships and collaborations worldwide, while the secondary research methodology identified all the major suppliers, distributors, and service providers functioning in the target market. While interviewing, the respondents were also inquired about their competitors.
To get a copy of the sample report, click here @ https://straitsresearch.com/report/Pharmacovigilance-and-Drug-Safety-Software-Market/request-sample
Pharmacovigilance and Drug Safety Software Report Highlights:
Market Dynamics – Trends, Drivers, Restraints
SWOT Analysis
Distribution Channel Analysis
Import/Export Strategies
Product Bench-marking and Pricing Analysis of Key Industry Players
Policy and Regulation.
Reasons to Go For this Report:
To study and evaluate the overall size of the market, as well as the aggregate share, in terms of value and volume.
To identify and forecast the global market based on product type, application, end-user, technology, industry vertical, and region/country.
To determine the driving and restraining factors, challenges, threats, and lucrative opportunities for the market.
To determine and study the profile of major Pharmacovigilance and Drug Safety Software industry participants.
To conduct the pricing analysis for the global Pharmacovigilance and Drug Safety Software market.
To study the competitive developments such as expansions, new product launches, and mergers & acquisitions in the market.
Lastly, the report presents statistics, market current trends, and end-user applications. The research conclusions, findings, appendix, and data source with a summarized view of the Pharmacovigilance and Drug Safety Software market 2019 is also included in this report.
To read a detailed description of the report, click here @ https://straitsresearch.com/report/Pharmacovigilance-and-Drug-Safety-Software-Market
If You Need More Information regarding this Pharmacovigilance and Drug Safety Software Market, Please Drop a mail on sales@straitsresearch.com OR Call on +1 6464807505 (US)
For more details, please contact us -
Email: sales@straitsresearch.com
Address: 825 3rd Avenue, New York, NY, USA, 10022
Tel: +1 6464807505, +44 203 318 2846
Website: https://straitsresearch.com/
About Us:- Straits Research is a leading market research and market intelligence organization, specializing in research, analytics, and advisory services along with providing business insights & market research reports.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance and Drug Safety Software Market Will Grow During Forecasts Period 2019-2026 here
News-ID: 1881176 • Views: …
More Releases from Straits Research
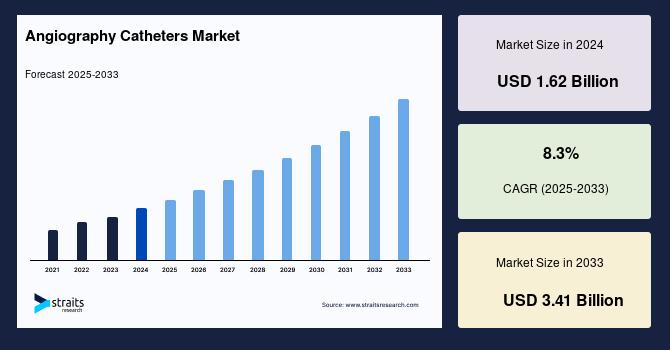
Angiography Catheters Market Size to Reach USD 3.41 Billion by 2033, Driven by R …
The global angiography catheters market is experiencing strong momentum, driven by the growing burden of cardiovascular diseases (CVDs), rising preference for minimally invasive diagnostic and interventional procedures, and ongoing innovations in catheter-based imaging technologies. Industry estimates indicate that the market is expected to expand from USD 1.8 billion in 2025 to USD 3.41 billion by 2033, progressing at a compound annual growth rate (CAGR) of 8.3% over the forecast period.
Angiography…
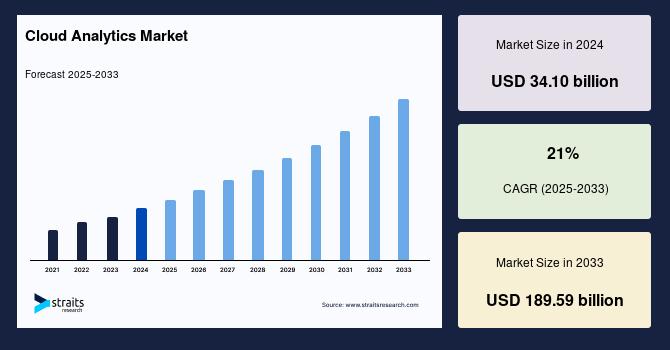
Cloud Analytics Market Size Set to Surge to USD 189.59 Billion by 2033 | Massive …
The global cloud analytics market is poised for exceptional growth as organisations leverage the power of the cloud to collect, analyse and visualise large volumes of data for actionable business insights. According to recent research, The global cloud analytics market size was worth USD 34.10 billion in 2024 and is estimated to reach an expected value of USD 189.59 billion by 2033, growing at a CAGR of 21% during the…
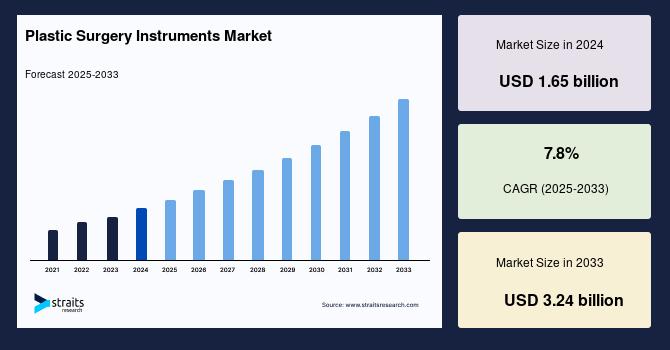
Plastic Surgery Instruments Market Size to Reach USD 3.24 Billion by 2033 | Glob …
The global plastic surgery instruments market is witnessing robust expansion, driven by the rising demand for cosmetic and reconstructive surgeries worldwide. According to a new study by Straits Research, the market size is estimated at USD 1.78 billion in 2025 and is projected to reach USD 3.24 billion by 2033, reflecting a compound annual growth rate (CAGR) of 7.8% during the forecast period (2025-2033).
The rising popularity of aesthetic enhancement procedures…
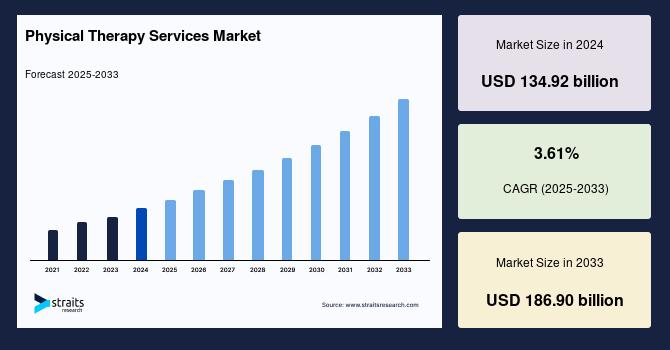
Physical Therapy Services Market Outlook 2025-2033: Rise of Home-Based Care and …
The global physical therapy services market is witnessing significant expansion, fueled by the growing prevalence of chronic diseases, increasing sports-related injuries, and technological innovations such as tele-rehabilitation and AI-based therapy platforms. According to Straits Research, the global market size is estimated at USD 140.69 billion in 2025 and is projected to reach USD 186.90 billion by 2033, exhibiting a steady CAGR of 3.61% during the forecast period.
Read the full report…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…
