Press release
U.S. Biosimilars Market for biologic medical product to reach US$ 17,696.0 Mn units by 2026
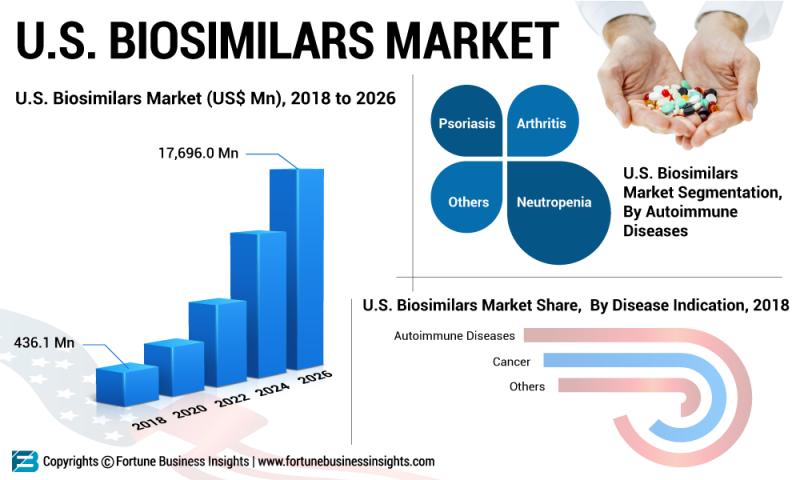
The U.S. Biosimilars Market will gain momentum from the increasing government support to adopt biosimilars in the healthcare sectors. According to a report by Fortune Business Insights, titled, “Biosimilars: U.S. Market Analysis, Insights and Forecast, 2019-2026,” The U.S. Biosimilars Market is projected to reach US$ 17,696.0 Mn by 2026. The report provides valuable insights into the drivers influencing growth of the market. As per the report, The U.S. Biosimilars Market is anticipated to report an impressive CAGR of 54.7% during the forecast period. The report also states that the U.S. market was valued at US$ 436.1 Mn in 2018.Browse Complete Report Details@ https://www.fortunebusinessinsights.com/industry-reports/u-s-biosimilars-market-100990
Some of the other players operating in the U.S. biosimilars market are
• F. Hoffmann-La Roche Ltd.,
• AbbVie Inc.,
• Merck & Co.,
• Celltrion Inc.,
• Coherus BioSciences, Inc.,
• Teva Pharmaceutical Industries Ltd.,
• Eli Lilly and Company
Pfizer Inc., Samsung Bioepis Co. Ltd., and Other Key Players Tend to Focus on Product Launch to Strengthen Position
Samsung Bioepis Co. Ltd, a biopharmaceutical company, received approval from Food and Drugs Administrations for ETICOVO, a biosimilar of Etanercept in April 2019. It is used for the treatment of plaque psoriasis, rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, ankylosing spondylitis, and psoriatic arthritis. Pfizer Inc. a multinational pharmaceutical company based in the U.S., received approval from FDA for TRAZIMERA, a biosimilar referencing Herceptin in March 2019. It will be used to treat gastroesophageal junction adenocarcinoma and breast cancer.
Order Full Report@ https://www.fortunebusinessinsights.com/checkout-page/100990
Autoimmune Diseases Segment Projected to Dominate the Market in the U.S.
The U.S. biosimilars market is classified into three segments, namely, by distribution channel, by disease indication, and by drug class. By distribution channel, the market is grouped into online pharmacy, hospital pharmacy, and retail pharmacy. In terms of disease indication, the market is divided into cancer and autoimmune diseases such as arthritis, neutropenia, psoriasis, and others. In 2018, the autoimmune diseases held a market share of 92.0%. The segment is anticipated to remain in the leading position the U.S. biosimilars market during the forecast period. Factors like increasing adoption of biosimilars, rise in the prevalence of autoimmune diseases, and availability of limited drugs are likely to boost the segment. The Centers of Disease Control and Prevention declared that approximately 26% adults are estimated to have arthritis by 2040. This in turn, will increase the demand for biosimilars to treat arthritis. Moreover, treatment of cancer is expensive in the U.S. Combined with this, constant investments in research and development and rise in the adoption of sedentary lifestyle are projected to favor growth of the cancer segment throughout the forecast period in the U.S.
Segmentation
By Drug Class
· Filgrastim & Pegfilgrastim
· Monoclonal Antibodies
· Others
By Disease Indication
· Cancer
· Autoimmune Diseases
· Arthritis
· Psoriasis
· Neutropenia
· Others
· Others
By Distribution Channel
· Hospital Pharmacy
· Retail Pharmacy
· Online Pharmacy
About Us:
Fortune Business Insights offers expert corporate analysis and accurate data, helping organizations of all sizes make timely decisions. We tailor innovative solutions for our clients, assisting them address challenges distinct to their businesses. Our goal is to empower our clients with holistic market intelligence, giving a granular overview of the market they are operating in.
Our reports contain a unique mix of tangible insights and qualitative analysis to help companies achieve sustainable growth. Our team of experienced analysts and consultants use industry-leading research tools and techniques to compile comprehensive market studies, interspersed with relevant data.
At Fortune Business Insights we aim at highlighting the most lucrative growth opportunities for our clients. We therefore offer recommendations, making it easier for them to navigate through technological and market-related changes. Our consulting services are designed to help organizations identify hidden opportunities and understand prevailing competitive challenges.
Contact us:
Fortune Business Insights Pvt. Ltd.
308, Supreme Headquarters,
Survey No. 36, Baner,
Pune-Bangalore Highway,
Pune - 411045, Maharashtra, India.
Phone:
US :+1 424 253 0390
UK : +44 2071 939123
APAC : +91 744 740 1245
Email: sales@fortunebusinessinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release U.S. Biosimilars Market for biologic medical product to reach US$ 17,696.0 Mn units by 2026 here
News-ID: 1838428 • Views: …
More Releases from Fortune Business Insights Pvt. Ltd.

Global Bread Maker Market to Reach USD 11.13 Billion by 2032, Driven by a 4.71% …
The global bread maker market size was valued at USD 7.48 billion in 2023 and is expected to be worth USD 7.70 billion in 2024. The market is projected to reach USD 11.13 billion by 2032, recording a CAGR of 4.71% during the forecast period.
Bread maker machines enable users to create a diverse range of bread at home cost-effectively. The increasing demand for innovative home appliances and the rising replacement…

Streetwear Industry to Reach $637.12 Billion by 2032 at 7.89% CAGR During Foreca …
The global streetwear market size was valued at USD 325.28 billion in 2023. The market is projected to be worth USD 347.14 billion in 2024 and reach USD 637.13 billion by 2032, exhibiting a CAGR of 7.89% during the forecast period. Asia Pacific dominated the streetwear market with a market share of 36.17% in 2023.
Fortune Business InsightsTM displays this information in a report titled, "Streetwear Market, 2024-2032."
Request a Free Sample…

Streetwear Market Size, Share, Analysis, Overview, Demand, Report, 2032 | Compan …
The streetwear market size was valued at USD 325.28 billion in 2023 and is expected to be worth USD 347.14 billion in 2024. The market is projected to reach USD 637.13 billion by 2032, recording a CAGR of 7.89% during the forecast period.
Streetwear is youth-inspired clothing that is highly popular among hip-hop enthusiasts and skateboarders. It is popular for its vibrant colors, bold logo graphics, and unconventional designs. Streetwear is…

Perfume Market Size, Share, Growth, Demand, Overview, Report, 2032 | Companies- …
The global perfume market size was valued at USD 48.05 billion in 2023 and is projected to grow from USD 50.45 billion in 2024 to USD 77.52 billion by 2032, exhibiting a CAGR of 5.51% during the forecast period.
Perfumes are pleasant smelling solutions made by using oils, fragrances, and other ingredients to create a pleasing aroma. Increasing demand for high-quality beauty and grooming products globally is expected to boost the…
More Releases for Biosimilars
Transformative Trends Impacting the Biosimilars Market Landscape: Innovative Pro …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Biosimilars Market Size Valuation Forecast: What Will the Market Be Worth by 2025?
The dimensions of the biosimilars market have been rapidly expanding over the last couple of years. The escalation, from a worth of $18.65 billion in 2024 to an estimated value of $21.95 billion in 2025, denotes…
Evolving Market Trends In The Biosimilars Industry: Innovative Product Launched …
The Biosimilars Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Biosimilars Market Size During the Forecast Period?
The dimension of the biosimilars market has experienced substantial expansion in the last few years. The market value, which was at $18.65 billion in 2024,…
Prominent Rituximab Biosimilars Market Trend for 2025: Collaborative Innovations …
Which drivers are expected to have the greatest impact on the over the rituximab biosimilars market's growth?
The rituximab biosimilars market is anticipated to grow due to the projected increase in non-Hodgkin's lymphoma (NHL) cases. NHL is a cancer that originates in the white blood cells and lymphocytes, which are integral parts of the body's immune system. For example, the American Cancer Society, a cancer advocacy group based in the US,…
Global Oncology Biosimilars Market | Global Oncology Biosimilars Industry | Onco …
The oncology biosimilars market involves of sales of medicine and drug interrelated products for cancer treatment. Biosimilars are pharmaceuticals which are produced using cell lines and are fashionable to the manufacturer. The manufacturing of such cell line processes is a multipart and time-consuming procedure.
According to the report analysis, ‘Oncology Biosimilars Market Global Report 2020-30’ states that the worldwide oncology biosimilars market was worth USD 2990.34 million in 2019. It is…
Global Biosimilars Market | Global Biosimilars Industry | Global Biosimilars Mar …
The biosimilars market involves of sales of biosimilars and associated services that are cast-off to treat chronic sicknesses such as diabetes, arthritis, and cancer. The Biosimilars are pharmaceuticals that are produced using cell lines and offers no clinical difference as linked to biologics. The Biosimilars are made once the patent of biologics is deceased.
According to the report analysis, ‘Biosimilars Market Global Report 2020-30’ states that the worldwide biosimilars market was…
Insulin Biosimilars Market, by Biosimilar Type Rapid-acting Biosimilars, Long-ac …
Diabetes is a group of metabolic disease characterized by high blood sugar level due to inadequate secretion of insulin. Common symptoms of diabetes include increased hunger, tiredness, weight loss, and excessive thirst and urination. The prevalence of diabetes is increasing, in turn, boosting demand for insulin biosimilar. For instance, according the World Health Organization (WHO) report in 2014, globally around 422 million adults were living with diabetes and 1.5 million…