Press release
joimax® Starts Official Sale of its CE-Approved EndoLIF® Product Product Line
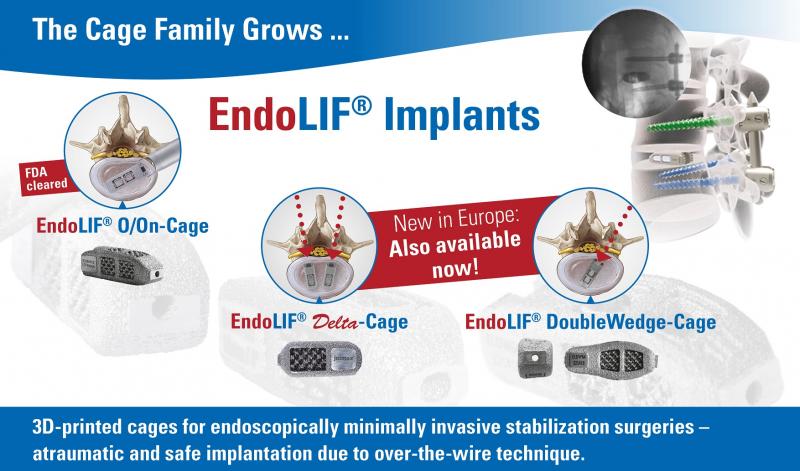
Karlsruhe, GER –joimax®, the Germany-based market leader of technologies and training methods for full-endoscopic minimally-invasive spinal surgery, announces the official sales launch of its complete, CE-approved, EndoLIF® product line. EndoLIF® is an instrument set for endoscopic-assisted minimally invasive lumbar interbody fusion. As already announced and showcased at earlier events, the EndoLIF® family consists of three devices: the well-known, FDA-approved, EndoLIF® O/On-Cage; the EndoLIF® Delta Cage; and the latest, EndoLIF® Double Wedge Cage — now, all widely available.All EndoLIF® products are manufactured using a 3D-printing process, which ensures an open diamond cell structure. The cages are safely implanted with a guide wire and are fillable with bone or bone substitute material. With their rough and porous surface, the implants guarantee optimal bone ingrowth, stability, and fusion.
The EndoLIF® product line provides the right implant for each patient and is an optimal solution for endoscopic spinal fusion. With the gentle and atraumatic access via gradual tissue dilatation, muscles remain intact, leaving important biomechanical structures in place after surgery.
As with any endoscopic procedure compared to conventional open-surgery, the benefits and fusion advantages are fully realized with the EndoLIF® program. Plus, by using the EndoLIF® instrument set, all three EndoLIF® cages can be implemented.
Dr. Florian Zentz (Munich, GER) emphasizes that there is an “extraordinarily high primary stability of the EndoLIF® DoubleWedge-Cage.” He notes that “after insertion, the cage sits very firmly in the intervertebral disc space and adjusts the angulation up to 18 degrees, almost directly enabling an optimal lordosis correction.”
Dr. Ralf Wagner (Frankfurt, GER) is also convinced of the notable advantages: “The Delta Cage is ideal for the posterior lumbar interbody fusion (PLIF) offering a gentle posterior alternative, especially at level L5-S1, with its large interlaminar window.”
Press Contact Germany:
joimax® GmbH
Amalienbadstr. 41 / RaumFabrik 61
76227 Karlsruhe / Germany
Sabine Jarosch
sabine.jarosch@joimax.com
0049 721 25514 0
Press Contact USA:
joimax® Inc.
Emily Campbell
emily.campbell@joimaxusa.com
001 949 859 3472
About joimax®
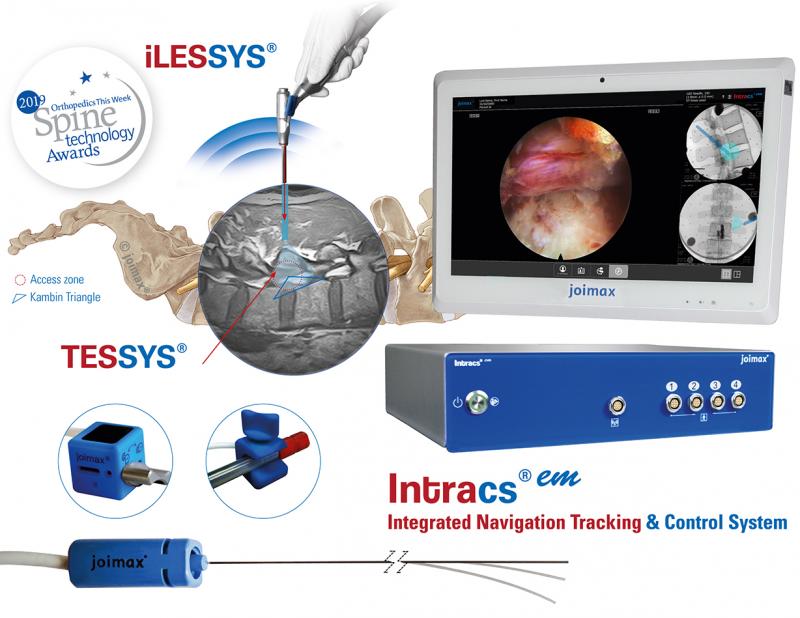
Founded in Karlsruhe, Germany, in 2001, joimax® is the leading developer and marketer of complete systems for full-endoscopic and minimally invasive spinal surgery. With the Endoscopic Surgical Systems TESSYS® (transforaminal), iLESSYS® (interlaminar) and CESSYS® (cervical) for decompression procedures, MultiZYTE® for facet and sacroiliac joint pain treatment and EndoLIF® and Percusys® for minimally-invasive endoscopically assisted stabilizations, established systems are provided, addressing a whole range of indications.
In procedures for herniated disc, stenosis, pain therapy or spinal stabilization treatment, surgeons utilize joimax® technologies to operate through small incisions under local or full anesthesia, via tissue and muscle-sparing corridors and through natural openings in the spinal canal, e.g. the intervertebral foramen, the so-called “Kambin triangle”.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release joimax® Starts Official Sale of its CE-Approved EndoLIF® Product Product Line here
News-ID: 1812612 • Views: …
More Releases from joimax GmbH
joimax® Celebrates Resounding Success at the German Spine Society (DWG) Convent …
Karlsruhe (GER) / Irvine, CA – December 3, 2019 – joimax®, the Germany-based market leader of technologies and training methods for full-endoscopic minimally-invasive spinal surgery, celebrated resounding success at this year’s German Spine Society (DWG), held November 28 to 30 in Munich, Germany. joimax® presented its newest product innovations and hosted several informative sessions to rave reviews, exceeding expectations.
Among the products showcased was a new member of the joimax® TESSYS®…
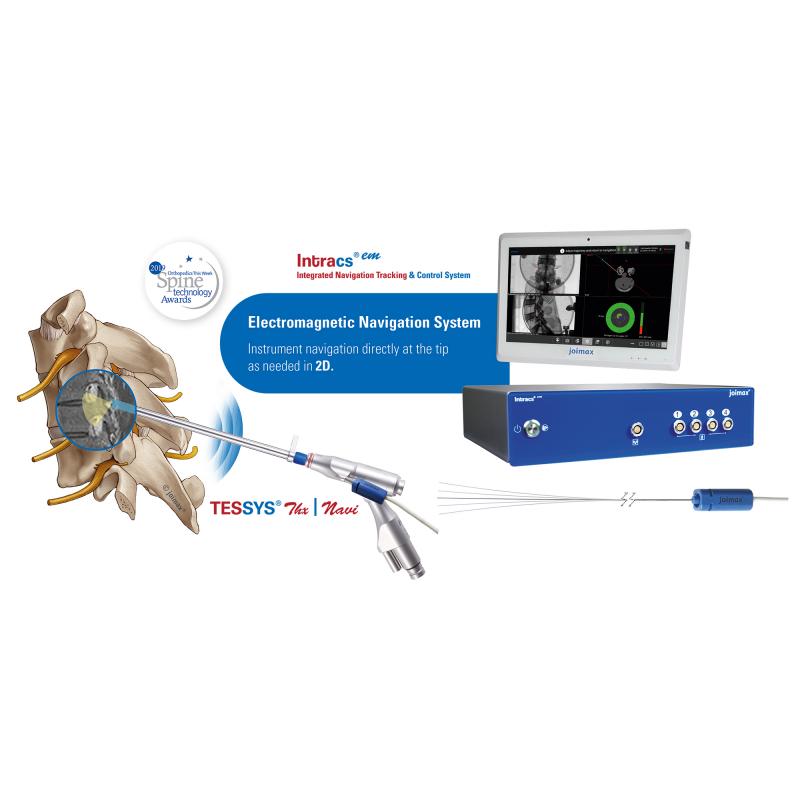
joimax® Wins 2019 Spine Technology Award for its Integrated Navigation Tracking …
Irvine, CA – joimax®, a leading developer and marketer of complete systems for endoscopic minimally invasive spinal surgery, is pleased to announce they’ve won a 2019 Orthopedics this Week Spine Technology Award for their new Integrated Navigation Tracking & Control System, Intracs®em. joimax® will be recognized with the award during the annual North American Spine Society Meeting (NASS), taking place September 25-28 in Chicago.
joimax® is heavily involved in this year’s…
More Releases for EndoLIF®
Punch!® Software Releases Punch!CAD™ SharkCAD® & ViaCAD® 15
NOVATO, Calif., December 10, 2024 - Punch!® Software, a leading software developer of Home Design and CAD titles for over 25 years, today announced the release of new Windows and Mac versions of the SharkCAD® 15 and ViaCAD® 15 family of products.
This latest release encompasses a range of variants for the Windows and Macintosh platform: SharkCAD® Pro, SharkCAD®, ViaCAD® Pro, ViaCAD® 2D/3D, and ViaCAD® 2D.
Punch!CAD™ products continue to provide innovative…
Cherrywork® Accounts Payable Automation by Incture® Now Available on SAP® Sto …
Incture® today announced the availability of Cherrywork® Accounts Payable Automation for purchase on SAP® Store, the online marketplace for SAP and partner offerings. Cherrywork® Accounts Payable Automation is built on SAP Business Technology Platform (SAP BTP) to streamline end-to-end accounts payable operations, reduce accounting costs, and help increase cash flow for customers.
"Cherrywork Accounts Payable Automation is the hyperautomation solution to step up the source to pay process for our customers.…
Ultimate Chemicals® Featured in Manufacturing Marvels® on The Fox Business Net …
MOORE, OK., Oct. 8, 2021/ -- Ultimate Chemicals® Inc., an integrated manufacturer and supplier of chemicals and related services, is pleased to announce it will be showcased in a broadcast of Manufacturing Marvels® scheduled to air on October 14, 2021 at approximately 9:30-9:44 p.m. EST on The Fox Business Network® (FBN).
Ultimate Chemicals has created their products to help restore surfaces to their optimum surface capability. Non-destructive to the surfaces to…
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA
Sunera Technologies, Inc Joins SAP® PartnerEdge® Delivering SAP® S/4HANA and Intelligent Enterprise to Manufacturing, Consumer Products, Higher education, and research, Industrial machinery and components, Life sciences and Automotive Industries
Chicago, IL, June 10, 2020 – Sunera Technologies, Inc announced that it had joined the SAP® PartnerEdge® program as a Re-sell & Services partner, through which it will resell SAP solutions to midsize organizations in North America. Through this engagement, SuneraTech…
American Megatrends Announces AMIBIOS®8 & Aptio®4.x Support for the Intel® Xe …
ATLANTA, GEORGIA - American Megatrends (AMI) is pleased to announce that its AMIBIOS®8 & Aptio®4.x products feature support for the new Intel® Xeon® 5500 Platform.
The Intel® Xeon® 5500 platform combines the Intel® Xeon® Processor 5500 Series with the Intel® 5520 Chipset for embedded, storage, security and communications infrastructure applications in a wide range of form factors. There are four different processor options for this platform with seven year lifecycle support…
CNUX .NET Providers for DB2® Earns IBM® DB2® Data Server Certification
CNUX Technologies Inc. announced today that CNUX .NET Providers for DB2® - Standard and Web Service Edition have achieved "Ready for IBM DB2 Data Server Software" status. With this designation CNUX customers can be assured that CNUX .NET Providers interacts and operates seamlessly with IBM® DB2® software.
The "Ready for IBM DB2 Data Server Software" program is a process designed for independent software vendors (ISVs) and solution integrators to validate their…