Press release
Biosimilars market has huge of CAGR of 49.1% during the forecast period 2020.
According to a new report published by Allied Market Research, titled, "World Biosimilars/Follow-On-Biologics - Market Opportunities, and Forecast, 2014-2020", the global Biosimilars/Follow-On-Biologics market is projected to grow at a CAGR of 49.1% from 2015 to 2020. Europe would continue to dominate the market while Asia Pacific would emerge as fastest growing region over the forecast period.Download the Sample Report @ http://bit.ly/2WKItRV
Recent approval of Zarxio (filgrastim-sndz) as first biosimilar by U.S. FDA has opened new opportunities for biosimilar manufactures. Patents for number of blockbuster bio-pharmaceuticals have either expired or are on the verge of expiration, which is majorly driving the growth of biosimilars industry. Changes in regulatory guidelines and convenient biosimilar drug approval processes have a major impact on the commercial growth of global biosimilars market. However, the high investment associated with research and development, longer development processes and requirements of economies of scale for profitability largely limit the growth of biosimilars market. The integration of developmental plan with regulatory guidelines and the adoption of optimal commercial strategies would play crucial role in commercial growth of biosimilars market.
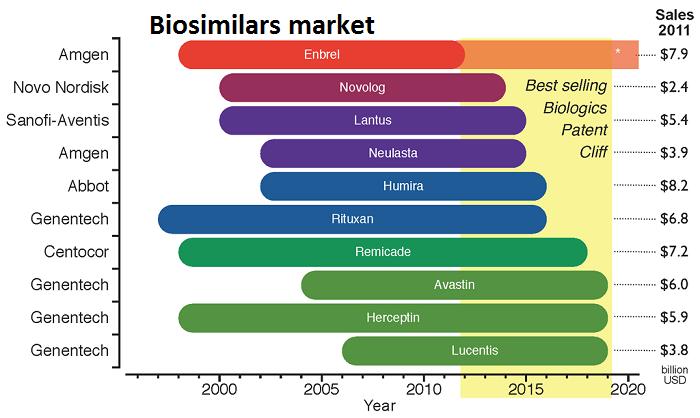
Glycosylated proteins namely, erythropoietin and monoclonal antibodies are the leading biosimilar segments commercially available across the globe, together accounting for about one-third of the market revenue in 2014. This significant hold in the market is chiefly due to its large application in the treatment of chronic conditions such as blood related disorders, cancer, among others.
Key findings of the study:
• The biosimilar applications in blood disorders and oncology collectively evaluated at 61% in 2014.
• Interferon is fastest growing biosimilar, expected to grow at a CAGR of 51.1% during the forecast period.
• U.S. would find space in biosimilar market with single-digit revenue share of overall biosimilar market by 2020.
For More Inquiry @ http://bit.ly/2KsuTMq
European region is the leading beneficiary of biosimilars market and is attracting investors, interested in biosimilars industry. The prospects in the European region are largely supplemented by the presence of streamlined regulatory guidelines and a high level of adoption for biosimilars among physicians. The establishment of favourable regulations such as Article 10(4) of Directive 2001/83/EC by the European Medical Association (EMA) has clarified the clinical aspect of biosimilars. This standard set by EMA has attracted investors to invest into the development of biosimilars. The Asia-Pacific region projects the fastest growth rate, supplemented by the high prevalence of chronic diseases and the growing demand for economic bio-pharmaceuticals. The commercial scenario of biosimilars in North America is bleak due to the absence of regulations for approval and commercialization. However, after the launch of the first biosimilar in United States, the investors are now focusing on this region to boost their prospects.
Contact Us:-
David Correa
5933 NE Win Sivers Drive
#205, Portland, OR 97220
United States
USA/Canada (Toll Free):
+1-800-792-5285, +1-503-894-6022, +1-503-446-1141
UK: +44-845-528-1300
Hong Kong: +852-301-84916
India (Pune): +91-20-66346060
Fax: +1(855)550-5975
help@alliedmarketresearch.com
Web: https://www.alliedmarketresearch.com
About Us:-
Allied Market Research (AMR) is a full-service market research and business consulting wing of Allied Analytics LLP based in Portland, Oregon. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions.” AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars market has huge of CAGR of 49.1% during the forecast period 2020. here
News-ID: 1764622 • Views: …
More Releases from Allied Market Research

Endpoint Security Market Size Growing at 8.4% CAGR Reach USD 31.9 Billion by 203 …
Allied Market Research published a new report, titled, "Endpoint Security Market Size Growing at 8.4% CAGR Reach USD 31.9 Billion by 2031." The report offers an extensive analysis of key growth strategies, drivers, opportunities, key segments, Porter's Five Forces analysis, and competitive landscape. This study is a helpful source of information for market players, investors, VPs, stakeholders, and new entrants to gain a thorough understanding of the industry and determine…
Smart Manufacturing Market Size Growing at 13.7% CAGR Reach USD 860 Billion by 2 …
Allied Market Research published a new report, titled, "Smart Manufacturing Market Size Growing at 13.7% CAGR Reach USD 860 Billion by 2031." The report offers an extensive analysis of key growth strategies, drivers, opportunities, key segments, Porter's Five Forces analysis, and competitive landscape. This study is a helpful source of information for market players, investors, VPs, stakeholders, and new entrants to gain a thorough understanding of the industry and determine…
Data Virtualization Market Sizze Growing at 21.7% CAGR Reach USD 22.2 Billion by …
According to the report published by Allied Market Research, Data Virtualization Market Sizze Growing at 21.7% CAGR Reach USD 22.2 Billion by 2031. The report provides an extensive analysis of changing market dynamics, major segments, value chain, competitive scenario, and regional landscape. This research offers valuable able guidance to leading players, investors, shareholders, and startups in devising strategies for sustainable growth and gaining a competitive edge in the market.
Driving Factors…

Europe IoT Market Growing at 19.0% CAGR Reach USD 12.30 Billion by 2031
According to the report published by Allied Market Research, Europe IoT Market Growing at 19.0% CAGR Reach USD 12.30 Billion by 2031. The report provides an extensive analysis of changing market dynamics, major segments, value chain, competitive scenario, and regional landscape. This research offers valuable able guidance to leading players, investors, shareholders, and startups in devising strategies for sustainable growth and gaining a competitive edge in the market.
The Europe IoT…
More Releases for Biosimilars
Transformative Trends Impacting the Biosimilars Market Landscape: Innovative Pro …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Biosimilars Market Size Valuation Forecast: What Will the Market Be Worth by 2025?
The dimensions of the biosimilars market have been rapidly expanding over the last couple of years. The escalation, from a worth of $18.65 billion in 2024 to an estimated value of $21.95 billion in 2025, denotes…
Evolving Market Trends In The Biosimilars Industry: Innovative Product Launched …
The Biosimilars Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Biosimilars Market Size During the Forecast Period?
The dimension of the biosimilars market has experienced substantial expansion in the last few years. The market value, which was at $18.65 billion in 2024,…
Prominent Rituximab Biosimilars Market Trend for 2025: Collaborative Innovations …
Which drivers are expected to have the greatest impact on the over the rituximab biosimilars market's growth?
The rituximab biosimilars market is anticipated to grow due to the projected increase in non-Hodgkin's lymphoma (NHL) cases. NHL is a cancer that originates in the white blood cells and lymphocytes, which are integral parts of the body's immune system. For example, the American Cancer Society, a cancer advocacy group based in the US,…
Global Oncology Biosimilars Market | Global Oncology Biosimilars Industry | Onco …
The oncology biosimilars market involves of sales of medicine and drug interrelated products for cancer treatment. Biosimilars are pharmaceuticals which are produced using cell lines and are fashionable to the manufacturer. The manufacturing of such cell line processes is a multipart and time-consuming procedure.
According to the report analysis, ‘Oncology Biosimilars Market Global Report 2020-30’ states that the worldwide oncology biosimilars market was worth USD 2990.34 million in 2019. It is…
Global Biosimilars Market | Global Biosimilars Industry | Global Biosimilars Mar …
The biosimilars market involves of sales of biosimilars and associated services that are cast-off to treat chronic sicknesses such as diabetes, arthritis, and cancer. The Biosimilars are pharmaceuticals that are produced using cell lines and offers no clinical difference as linked to biologics. The Biosimilars are made once the patent of biologics is deceased.
According to the report analysis, ‘Biosimilars Market Global Report 2020-30’ states that the worldwide biosimilars market was…
Insulin Biosimilars Market, by Biosimilar Type Rapid-acting Biosimilars, Long-ac …
Diabetes is a group of metabolic disease characterized by high blood sugar level due to inadequate secretion of insulin. Common symptoms of diabetes include increased hunger, tiredness, weight loss, and excessive thirst and urination. The prevalence of diabetes is increasing, in turn, boosting demand for insulin biosimilar. For instance, according the World Health Organization (WHO) report in 2014, globally around 422 million adults were living with diabetes and 1.5 million…