Press release
mivenion targets musculoskeletal imaging experts at ECR 2011
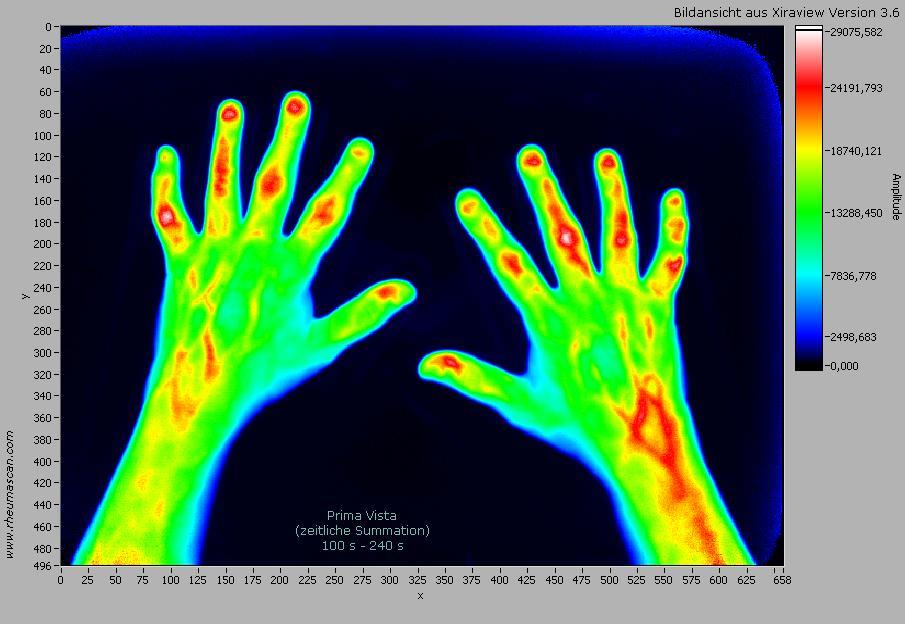
mivenion GmbH, the leading company in optical imaging of arthritis, will exhibit its groundbreaking Rheumascan workstation Xiralite® X4 and the most advanced XiraView 3.6 software for diagnostic imaging of inflammation in the hands at the forthcoming annual European Congress of Radiology (ECR) in Vienna, Austria, March 4-7. The ECR is one of the largest medical meetings worldwide and the second largest radiological congress, with around 19,000 participants from all over the world. Parallel to the Xiralite workstation, mivenion will feature key results of clinical studies using the system in comparison with clinical exam, power Doppler ultrasound, and contrast-enhanced MRI.Xiralite® X4 induces the fluorescence of ICG using light-emitting diodes (LED) and quantitatively records the fluorescence emitted in all joints of both hands. Thus active inflammation can be simultaneously diagnosed in up to 30 joints. Signal differences usually result from different "wash-in"/ "wash out" kinetics of the contrast agent at the site of inflammation. The fluorescence camera system Xiralite® X4 is optimized to detect even very low amounts of the contrast agent ICG and thereby enables the diagnosis of small inflammatory lesions. Signal intensity, localization of the signal, and the time period of enhancement are relevant for differential diagnosis. The procedure is suited for detection of early onset of inflammation in joints, as well as for monitoring of antiinflammatory therapies. The differential diagnosis of different diseases is a further application of Rheumascan.
“Rheumascan using the most advanced software version XiraView 3.6 reliably detects clinically active inflammation in the hands. Very surprisingly, we demonstrated a high specificity in healthy volunteers where almost no pathologic signal intensities are present. I personally believe that Rheumascan will be the future standard to exclude active disease in patients with unclear joint pain, to identify early arthritis, to discriminate between inflammatory and non-inflammatory pain disorders, and potentially to follow disease activity and evaluate anti-inflammatory therapeutic outcome” as stated by Dr. Malte Bahner, radiologist and responsible for clinical development at mivenion GmbH.
About mivenion:
mivenion GmbH is a life-science company focusing on personalized medicine for patients with inflammatory and autoimmune diseases. With its strong expertise in both pharmaceuticals and medical devices, mivenion is providing cutting edge solutions for life-long patient care. A compelling network of academia and industry partners is supporting mivenion in achieving its goals. Broad patent applications are the foundation of mivenion’s proprietary position. mivenion’s drug development candidates comprise both diagnostic and therapeutic properties and are perfectly suited for optimizing each individual treatment case. mivenion’s medical solutions encompass innovative diagnostic imaging and provide evidence on treatment outcome. Xiralite, the leading product of mivenion, enables the Rheumascan procedure. It for the first time provides an integrated solution for patients with inflammatory diseases in the hands. Based on optical technologies, active inflammation is diagnosed at a very early stage with confidence, attractive technical ease, and high patient comfort.
Contact:
Dr. Michael Schirner
mivenion GmbH
Robert-Koch-Platz 4
10115 Berlin
Germany
Phone +49 30 68837920
info@mivenion.com
www.mivenion.com
www.xiralite.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release mivenion targets musculoskeletal imaging experts at ECR 2011 here
News-ID: 163347 • Views: …
More Releases from mivenion GmbH
mivenion receives market authorization for Xiralite® in Canada
Fluorescence optical imaging (FOI) with the Xiralite® fluorescence imaging system X4 is a sensitive and safe medical procedure for visualization of altered microcirculation in the hands in early and late stages of different diseases such as rheumatoid arthritis, psoriatic arthritis, scleroderma, osteoarthritis or Raynaud’s syndrom. Xiralite® is CE marked and already established in five European countries. The whole procedure is highly standardized, very sensitive, fast, and convenient for the patients.…
mivenion announces 4 abstracts for fluorescence optical imaging using the Xirali …
Highlights include:
• ICG-enhanced fluorescence optical imaging (FOI) is able to detect typical inflammatory changes in subjects with arthralgia and psoriasis prior to signs of clinically established arthritis. The detection of typical inflammatory changes in subjects with cutaneous manifestations of psoriasis or family history of psoriasis and joint pain but without clinical signs of synovitis or tendinitis suggests that a non-arthritic, subclinical disease stage may precede the onset of clinically active…
mivenion to establish Rheumascan at Karolinska Institutet, Stockholm, Sweden
About 5% of the patients attending primary care physicians intermittently suffer from swelling and tenderness of the hand joints. In many cases, inflammatory joint diseases such as rheumatoid arthritis or psoriatic arthritis are the main cause of the disease symptoms and, without treatment, may lead to poor outcome and lifelong suffering. In recent years there is increasing evidence that early diagnosis and treatment of arthritis may effectively stop the progression…
mivenion receives ISO 13485 certification for Xiralite® manufacturing and distr …
mivenion has received the official ISO 13485:2003 + AC:2009 certification as a medical device manufacturer. Published in 2003, the ISO 13485 standard outlines the requirements for a comprehensive quality management system for all aspects of the design and manufacturing process for medical devices. The certification covers the design, development, manufacturing, and distribution of the patented Xiralite® fluorescence camera system and its XiraView® software.
“We are delighted that we can announce…
More Releases for Rheumascan
mivenion receives market authorization for Xiralite® in Canada
Fluorescence optical imaging (FOI) with the Xiralite® fluorescence imaging system X4 is a sensitive and safe medical procedure for visualization of altered microcirculation in the hands in early and late stages of different diseases such as rheumatoid arthritis, psoriatic arthritis, scleroderma, osteoarthritis or Raynaud’s syndrom. Xiralite® is CE marked and already established in five European countries. The whole procedure is highly standardized, very sensitive, fast, and convenient for the patients.…
mivenion announces 4 abstracts for fluorescence optical imaging using the Xirali …
Highlights include:
• ICG-enhanced fluorescence optical imaging (FOI) is able to detect typical inflammatory changes in subjects with arthralgia and psoriasis prior to signs of clinically established arthritis. The detection of typical inflammatory changes in subjects with cutaneous manifestations of psoriasis or family history of psoriasis and joint pain but without clinical signs of synovitis or tendinitis suggests that a non-arthritic, subclinical disease stage may precede the onset of clinically active…
mivenion to establish Rheumascan at Karolinska Institutet, Stockholm, Sweden
About 5% of the patients attending primary care physicians intermittently suffer from swelling and tenderness of the hand joints. In many cases, inflammatory joint diseases such as rheumatoid arthritis or psoriatic arthritis are the main cause of the disease symptoms and, without treatment, may lead to poor outcome and lifelong suffering. In recent years there is increasing evidence that early diagnosis and treatment of arthritis may effectively stop the progression…
mivenion receives ISO 13485 certification for Xiralite® manufacturing and distr …
mivenion has received the official ISO 13485:2003 + AC:2009 certification as a medical device manufacturer. Published in 2003, the ISO 13485 standard outlines the requirements for a comprehensive quality management system for all aspects of the design and manufacturing process for medical devices. The certification covers the design, development, manufacturing, and distribution of the patented Xiralite® fluorescence camera system and its XiraView® software.
“We are delighted that we can announce…
mivenion GmbH and PTB Berlin report patent grants for imaging of inflammation
mivenion GmbH announces that it has secured patent protection for its key technology of optical imaging of inflammation. The patent rights covering diagnostic technologies for the optical rheumatoid arthritis imaging were exclusively licensed from the Physikalisch-Technische Bundesanstalt (PTB Berlin). Now, mivenion and PTB Berlin have been granted its patent by the U.S. Patent and Trademark Office in February 2011, followed by a grant by the European Patent Office in December…