Press release
Orphan Drugs Market Growth in the United States has Risen Dramatically - Industry Forecast, 2016-2022
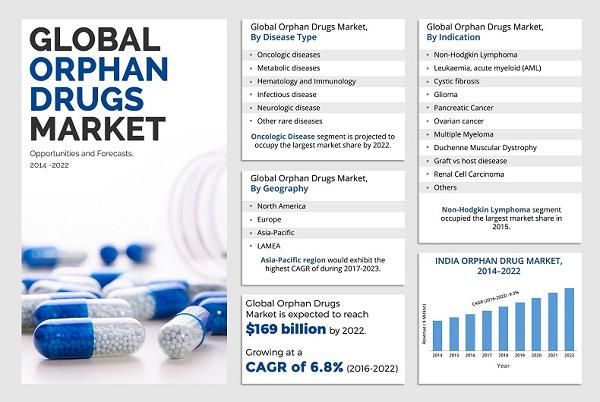
The major companies in Orphan Drugs Industry include AbbVie Inc., Aegerion Pharmaceuticals, Inc., Bristol-Myers Squibb Company, Celgene Corporation, F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc, Johnson & Johnson, Novartis AG, Pfizer Inc., and Sanofi.Global Orphan Drugs Market is expected to garner $169 billion from $106 billion and register a CAGR of 6.8% during the forecast period, 2016-2022. Orphan drugs form a niche yet considerably profitable segment of the pharmaceutical industry. Furthermore, the market exclusivity periods enjoyed by these drugs owing to favorable government legislations especially in the U.S. and Europe add to their value and profitability.
Download PDF Report Sample @ https://www.alliedmarketresearch.com/request-sample/204
Orphan drugs are specialized pharmaceutical agents that are administered for treatment of rare (orphan) diseases. These diseases have a very low prevalence rate, hence, pharmaceutical companies do not readily invest in these drugs as the returns on investment in orphan drugs is risky when compared with non-orphan drugs. Moreover, multiple clinical trials for drug testing cannot be voluntarily performed due to the small patient population. However, orphan drugs have shown tremendous potential in diagnosis and treatment of cancer, this trend is expected to continue throughout the forecast period. In addition, the increase in indications of orphan drugs to treat an array of different diseases such as lymphoma, leukemia, myeloma, and others boost the market growth.
The major factors that drive the global orphan drugs market are conducive government regulations, market exclusivity for orphan drugs, and surge in prevalence of rare diseases. In addition, growth in novel indications designated for known orphan drugs, and untapped emerging markets thereby provide lucrative opportunities for market growth. However, limited patient pool for clinical trial & product marketing and high treatment costs per patient restrain the market growth.
The oncologic disease type segment occupies the greatest share in the orphan drug market owing to the array of diverse forms of rare cancers, such as leukemia, myeloma, angiosarcoma, and others prevalent in the patient population. Whereas, the renal cell carcinoma indication is anticipated to grow at the highest rate. The growth for this segment is due to increase in investment in R&D throughout the world along with surge in awareness about kidney cancer. Multiple myeloma currently dominates the indication segment and is expected to grow at a CAGR of 7.8%.
In 2015, Asia-Pacific and LAMEA collectively accounted for around two-fifths of the global orphan drugs market and are expected to continue this trend due to rise in awareness regarding orphan drugs in China, India, and other developing economies. Various governmental and non-governmental organizations implement favorable legislations that supplement the growth of orphan drugs market. In addition, increase in investment in R&D for drug development by public and private sectors is also expected to boost the market growth.
Table Of Content
Chapter: 1 INTRODUCTION
1.1. REPORT DESCRIPTION
1.2. KEY BENEFITS
1.3. KEY MARKET SEGMENTS
1.4. RESEARCH METHODOLOGY
1.4.1. Primary research
1.4.2. Secondary research
1.4.3. Analyst tools and models
Chapter: 2 EXECUTIVE SUMMARY
2.1. CXO PERSPECTIVE
Chapter: 3 MARKET OVERVIEW
3.1. MARKET DEFINITION AND SCOPE
3.2. KEY FINDINGS
3.2.1. Top investment pockets
3.2.2. Top winning strategies
3.3. PORTER’S FIVE FORCES ANALYSIS
3.4. PATENT ANALYSIS.
3.4.1. Patent analysis by year (2010-2016)
3.4.2. Patent analysis by region
3.4.3. Patent analysis by indication
3.5. REGULATIONS
3.6. MARKET DYNAMICS
3.6.1. Drivers
3.6.1.1. Favorable government policies
3.6.1.2. Availability of exclusivity for orphan drugs
3.6.1.3. Surge in prevalence of rare diseases
3.6.2. Restraints
3.6.2.1. Limited patient pool for clinical trials and product marketing
3.6.2.2. High treatment costs per patient
3.6.3. Opportunities
3.6.3.1. Use of orphan drugs for novel conditions/indications
3.6.3.2. Untapped emerging markets
Access Full Summery @ https://www.alliedmarketresearch.com/orphan-drug-market
About us
Allied Market Research, a market research and advisory company of Allied Analytics LLP, provides business insights and market research reports to large as well as small & medium enterprises. The company assists its clients to strategize business policies and achieve sustainable growth in their respective market domain.
Allied Market Research provides one stop solution from the beginning of data collection to investment advice. The analysts at Allied Market Research dig out factors that help clients to understand the significance and impact of market dynamics. The company amplies client’s insight on the factors, such as strategies, future estimations, growth or fall forecasting, opportunity analysis, and consumer surveys among others. As follows, the company offers consistent business intelligent support to aid the clients to turn into prominent business firm.
Contact
David Correa
5933 NE Win Sivers Drive
#205, Portland, OR 97220
United States
Toll Free: +1-800-792-5285
UK: +44-845-528-1300
Hong Kong: +852-301-84916
India (Pune): +91-20-66346060
Fax: +1⟨855⟩550-5975
help@alliedmarketresearch.com
Web: www.alliedmarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Orphan Drugs Market Growth in the United States has Risen Dramatically - Industry Forecast, 2016-2022 here
News-ID: 1566411 • Views: …
More Releases from Allied Market Research

Bird Feeder Market is Anticipated to Develop Altogether at Strong CAGR; Aspects, …
Allied Market Research published a report, titled, "Bird Feeder Market by Type (Window Feeders, Tube Feeders, Hopper Feeders, Platform Feeders, Others), by Material (Metal, Plastic, Glass, Others), by Mount Type (Pole, Hanging, Window, Others), by Distribution Channel (Supermarkets and hypermarkets, Specialty Stores, Other Retail Stores, Online): Global Opportunity Analysis and Industry Forecast, 2021-2031." According to the report, the global bird feeder industry was pegged at $1.1 billion in 2021, and…

Aromatherapy Products Industry Performance Metrics: CAGR and USD Comparisons for …
According to a new report published by Allied Market Research, titled, "Aromatherapy Products Market," The aromatherapy products market was valued at $2.3 billion in 2021, and is estimated to reach $5.3 billion by 2031, growing at a CAGR of 9% from 2022 to 2031.
Aromatherapy is the technique of using essential oils (EOs) and other fragrant molecules from plants to influence someone's mood or health. It is frequently linked to complementary…

Women Sports and Swimwear Market Research Report: Unveiling CAGR and USD Project …
According to a new report published by Allied Market Research, titled, "Women Sports and Swimwear Market," The women sports and swimwear market size was valued at $81.73 billion in 2021, and is estimated to reach $148.32 billion by 2031, growing at a CAGR of 6.4% from 2022 to 2031.
➡️Download Research Report Sample & TOC : https://www.alliedmarketresearch.com/request-sample/17283
Sports clothing is typically worn during athletic competitions or during workouts. When performing physical movements,…
Doors Market to Reach $206.6 billion by 2031 at 5.2%: Allied Market Research
Allied Market Research published a report titled, "Doors Market by Type (Interior Doors, Exterior Doors), by Material (Wood, Glass, Metal, Plastic, Others), by Mechanism (Swing Doors, Sliding Doors, Folding Doors, Revolving Doors, Others): Global Opportunity Analysis and Industry Forecast, 2021-2031."
Drivers, Restraints and Opportunities
Increase in the trend of multifamily housing, rise in adoption of automated doors in commercial sector such as airports, malls, corporate offices, and others, development of energy efficient…
More Releases for Orphan
Acquired Orphan Blood Disease Market
Acquired Orphan Blood Disease Market to reach over USD 18.93 billion by the year 2031 - Exclusive Report by InsightAce Analytic
According to a new report by InsightAce Analytic, the "Acquired Orphan Blood Disease Market" in terms of revenue was estimated to be worth $8.65 billion in 2023 and is poised to reach $18.93 billion by 2031, growing at a CAGR of 10.47% from 2024 to 2031.
Get Free Access to…
Orphan Drugs Market Size to Hit $3199.3 Billion by 2028 | Orphan Drugs Industry …
Market Overview:
According to our experience research team, Orphan Drugs Market was valued at USD 112.36 Billion in 2021, and the global Orphan Drugs industry is projected to reach a value of USD 3199.3 Billion by 2028, at a CAGR of 7.4% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology, Energy &…
Orphan Drugs for Cancer Pipeline Analysis
A huge market opportunity is offered by small patient population which suffers from rare or orphan diseases. Among the category of new orphan drugs, Oncology account for the largest disease group in recent years. It has been observed that majority of the orphan drugs in the clinical stages are for rare cancer disease drugs, and are in the late stages of the pipeline. Some of the drugs are being developed…
US Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the pharmaceuticals industry.…
Europe Orphan Drugs Pipeline Analysis
“Europe Orphan Drugs Pipeline Analysis” by PNS Pharma gives comprehensive insight on the various drug profiles under Orphan Drugs status in Europe. Research report covers all the ongoing drug development in various phases. Each drug profiles include detailed information like: Originator, Owner, Collaborator, Technology Provider, Licensee, Development Phase, Development Indications, Mechanism of Action, Chemical Formula, Country of Development and detailed analysis on the development process. The information for particular drug…
Global Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US & Europe, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the…