Press release
Popularity of Molecular Diagnostic Testing to Offer Breakthrough Test Results in the US POC Diagnostic Market | Arizton
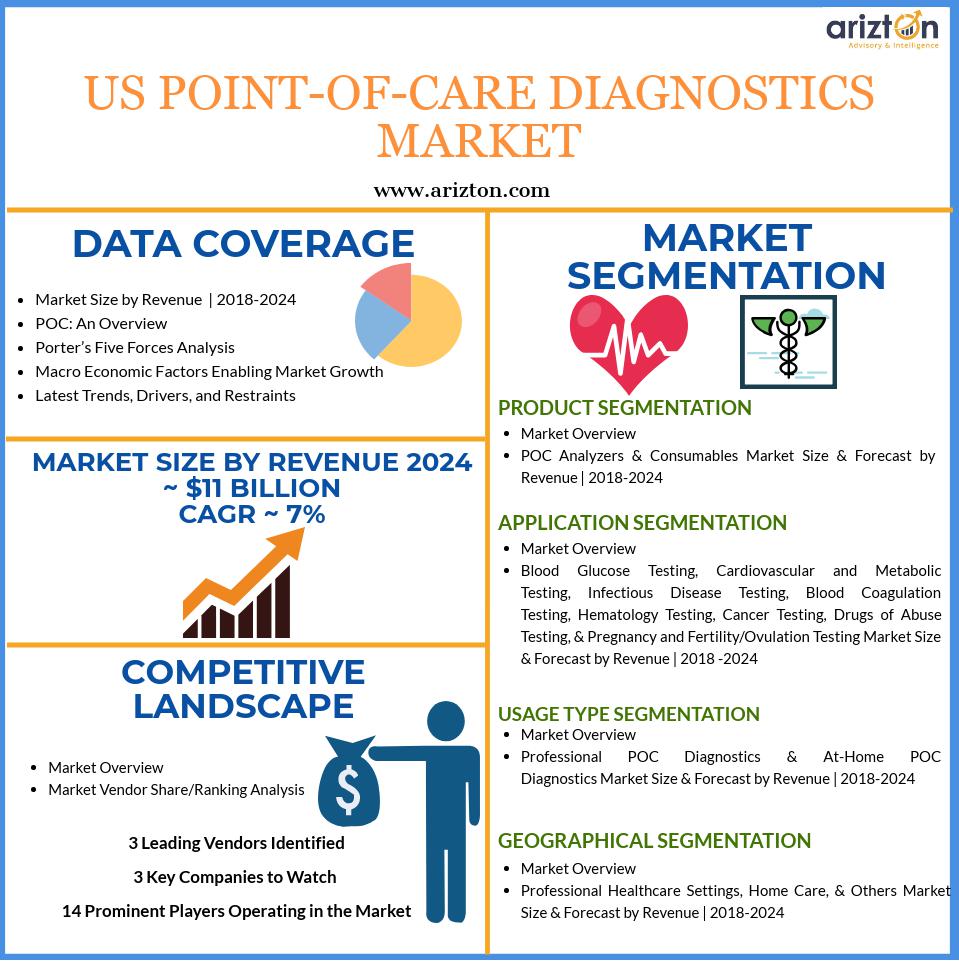
The paradigm shift from curative medicine to predictive, personalized, and pre-emptive medicine will drive the demand in the US POS diagnostic market.Arizton’s recent market research report on the US POC diagnostic market provides comprehensive industry analysis, trend forecasts, and competitive analysis. The research study segments the market by product (POC analyzers and consumables), by application (blood glucose testing, cardiovascular and metabolic testing, infectious disease testing, blood coagulation testing, hematology testing, cancer testing, drugs of abuse testing, and pregnancy and fertility/ovulation testing), by usage type (professional POC diagnostics and at-home POC diagnostics), by end-users (professional healthcare settings, home care, and others), and offers detailed competitive analysis.
The US POC diagnostic market is projected to reach values of approximately $11 billion by 2024, growing at a CAGR of more than 7% during 2018-2024.
The recent emergence of technological innovations in biosensors, lab-on-a-chip, smartphones, and wearable devices is contributing to the evolution of the US market. The paradigm shift from curative medicine to predictive, personalized, and pre-emptive medicine will drive the demand in the US POS diagnostic market.
Request for your free sample today!: https://www.arizton.com/market-reports/us-point-of-care-diagnostics-market
The top 3 drivers and trends augmenting the transformation of the US POC diagnostic market are discussed below:
Vendors Focus on Manufacturing Innovative POC Platforms for Existing and New Applications
The leading vendors are increasingly focusing on technological advancements and improvements is one of the major factors contributing to the growth of the US POC diagnostic market. The leading companies are leveraging technology for the development of highly advanced and innovative POC analyzers and rapid test kits in the US market. A broad range of acute and chronic diseases is encouraging players to develop advanced technological platforms, analyzers, test kits, and reagents in the market. Technological will revolutionize the molecular diagnostics, cancer testing, cardiac markers, infectious disease testing, urinalysis, and coagulation testing, thereby driving demand in the US POC diagnostic market. Many vendors are incorporating innovative technologies and platforms that are widely used in clinical diagnostic analyzers to develop innovative and compact POC analyzers and modalities that will attract new consumers in the market. The launch of advanced POC devices, benchtop diagnostic analyzers, and hand-held devices will augment the development of the US market over the next few years. The introduction of label-free biosensors, such as electrochemical, white light reflectance spectroscopy, and surface plasmon resonance will lead to the launch of next-generation devices in the US POC diagnostic market.
Growing Demand for POC Molecular Diagnostic Testing
The increasing demand for molecular diagnostic testing is fueling the growth of the US POC diagnostic market during the forecast period. The development of real-time PCR (qPCR)expanded the role of molecular diagnostics in the healthcare industry. The molecular diagnostic tests performed at POC setting or near the patients are resulting in breakthrough tests in recent years in the US market. Molecular testing canimprove the specificity and sensitivity of existing near-patient and rapid diagnostic tests, thereby propelling the growth of the US POC diagnostic market. The increasing application of this diagnostic testing across laboratory settings will boost the demand in the market. For instance, in September 2015, Roche Diagnostics received CLIA waiver status for the cobas Influenza A/B test for use on the cobas Liat system. The company claims that cobas Influenza A/B test was the first CLIA-waived real-time PCR test to detect influenza A and B less than 20 minutes. Such developments will result in automation of testing processes in the market. The introduction of these devices will result inreagent preparation, inhibitor removal, target enrichment, amplification, nucleic acid extraction, and real-time detection in the market. The growing demand for easy-to-use, compact, and portable devices will fuel the growth of the US POC diagnostic market.
Request for your free sample today!https://www.arizton.com/market-reports/us-point-of-care-diagnostics-market
High Growth Potential for Multiplexed POC Diagnostic Testing (xPOCT)
The growing requirement for the early and accurate diagnosis of a specific ailment that results in effective treatment will lead to the demand in the US POC diagnostic market. With POC testing immediate decision making in cases of sepsis or stroke, quick,and precise confirmation of clinical findings is becoming easier in the market. xPOCT is known as a simultaneous on-site measurement of different analytes from a single sample,and it is gaining immense popularity in the USPOC diagnostic market. The increasing use of xPOCT devices ensures the quality and performance requirements of in-vitro diagnostics by non-experts, specifically for physicians in ambulatory care settings in the market. The growing popularity of innovative home health monitoring systems and personalized medicine is driving the growth of the US market. The vendors are developing next-generation xPOCT systems with the combination of high-performance,andlow-system complexity features to gain a larger US POC diagnostic market share.
The leading vendors in the US POC diagnostic marketare Abbott, Roche Diagnostics, and Siemens Healthineers.
The complete overview of the latest market research report on US POC diagnostic market by Arizton is now available.
The report also offers a detailed study of major trends, drivers, challenges, and also provides the market size and forecast for major geographical regions and key countries.
Source: https://www.arizton.com/news/press-release/us-poc-diagnostic-market-trends-drivers
About Arizton Advisory & Intelligence
Arizton – Advisory and Intelligence is an innovation and quality-driven firm, which offers cutting-edge research solutions to clients across the world. We excel in providing comprehensive market intelligence reports and advisory and consulting services.
Arizton has gained a paramount standpoint in the market research arena as it offers top of the line solutions to clients to assess market landscape and to finalize foolproof business strategies. We are committed to provide inclusive market research reports and consulting services to clients from diversified industries including –Consumer Goods & Retail Technology, Automotive and Mobility, Smart Tech, Healthcare and Lifesciences, Industrial Machinery, Chemicals and Materials, IT and Media, Logistics and Packaging
Arizton comprises a team of exuberant and well-experienced analysts who have mastered in generating incisive reports. Our specialist analysts possess exemplary skills in market research. We train our team in advanced research practices, techniques, and ethics to outperform in fabricating impregnable research reports.
Arizton Advisory & Intelligence
Chicago, Illinois, 60605
Mail: enquiry@arizton.com
Call: +1-312-235-2040
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Popularity of Molecular Diagnostic Testing to Offer Breakthrough Test Results in the US POC Diagnostic Market | Arizton here
News-ID: 1480060 • Views: …
More Releases for POC
Primary Catalyst Driving POC Medical Imaging Market Evolution in 2025: Chronic D …
What market dynamics are playing a key role in accelerating the growth of the poc medical imaging market?
The escalating occurrence of long-term diseases is predicted to boost the expansion of the point-of-care medical imaging market in the future. The chronic disease prevalence pertains to the fraction or the count of people in a community affected by enduring health ailments at any given moment. This transition originates from lifestyle options, inherited…
Transforming the Point-of-Care (POC) Coagulation Testing Market in 2025: Cardiov …
"What Is the Expected Size and Growth Rate of the Point-of-Care (POC) Coagulation Testing Market?
The point-of-care (POC) coagulation testing market has grown significantly in recent years. It will grow from $1.18 billion in 2024 to $1.25 billion in 2025, at a CAGR of 5.8%. The growth is driven by increasing patient demand, the growing trend of point-of-care testing, high reliability in coagulation status testing, automation, integration with electronic health records…
POC Diagnostic Testing Devices | 1drop
1drop Inc.'s POC diagnostic testing devices are essential tools in modern healthcare, designed to deliver quick and accurate results for various health conditions at the point of care. These devices make diagnostics more accessible and timelier, enhancing healthcare delivery and patient outcomes.
Point-of-Care Molecular Diagnostic Platform
1drop offers a comprehensive POC molecular diagnostic platform that consists of three main components:
• Reagent: Specialized reagents formulated for specific tests, including molecular diagnostics.
• Device: The 1POT™ Duo…
POC Diagnostics Market Size 2024 to 2031.
Market Overview and Report Coverage
POC, or Point of Care, Diagnostics refer to medical tests that are performed outside of a traditional laboratory setting, allowing for quicker results and immediate treatment decisions. The POC Diagnostics Market encompasses a wide range of diagnostic tests including blood glucose monitoring, pregnancy tests, infectious disease testing, and more.
The future outlook of the POC Diagnostics Market is optimistic, with the market expected to grow…
Point of Care (POC) Coagulation Testing Devices Market - Transforming Coagulatio …
Newark, New Castle, USA: The "Point of Care (POC) Coagulation Testing Devices Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Point of Care (POC) Coagulation Testing Devices…
POC Cardiovascular Diagnostic Market 2022 | Detailed Report
According to Market Study Report, POC Cardiovascular Diagnostic Market provides a comprehensive analysis of the POC Cardiovascular Diagnostic Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Download FREE Sample Report @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=5467652
The report provides a comprehensive analysis of company profiles listed below:
- Abbott
- Biomerieux
- Danaher
- F.…