Press release
Organon Counts on PAS-X for their Production of Active Pharmaceutical Ingredients

Organon's state-of-the-art facility for the production of Active Pharmaceutical Ingredients (APIs) in Oss, The Netherlands
Lueneburg, Germany, January 17, 2007 – Organon, the human healthcare business unit of Akzo Nobel and one of the largest manufacturers of Active Pharmaceutical Ingredients (APIs) worldwide, has finished the installation of Werum Software & Systems’ PAS-X MES software in their new state-of-the-art API-powder processing production facility in Oss, The Netherlands.
Mixing and milling as well as a final-product warehouse were combined in this new factory. Organon uses PAS-X for recipe management, weighing and dispensing, shop floor management, electronic batch recording, and warehouse management. Throughout all the manufacturing processes PAS-X has replaced paper-based recipes and SOPs by electronic records. Operators do not only get information about the production processes on screen, but they are also able to give feedback about process parameters by computer.
Mixing and milling are part of the final activities in the overall manufacturing process of Active Pharmaceutical Ingredients. In this stage the final product is already present in a solid form, usually as a powder. Depending on customer specifications this powder must be homogenized and milled or micronized to make it yet finer. In the warehouse final products are received, stored, picked, weighed and either sent to external customers or to other Organon departments.
The primary goal pursued by the implementation of the PAS-X MES system was quality assurance through prevention of mistakes, registration and verification of critical activities and data, acquisition of deviations, and tracking and tracing of batches and equipment. Moreover, the MES system contributes to an improved efficiency through barcode scanning instead of manual entries; through detailed location tracking so that specific batches can be found and picked more easily; and through more effective creation and maintenance procedures for Master Batch Records by authorized employees.
The installation includes manual or computerized interfaces with various systems, such as ERP, Substance Information System, Document Management System (EDMS), and LIMS.
About Werum
Werum Software & Systems (www.werum.com) is a leading supplier of Manufacturing Execution Systems for the pharmaceutical and biopharmaceutical industry worldwide. Twelve of the world's top 20 pharmaceutical companies use Werum's product suite PAS X to run their manufacturing business. Outstanding PAS X features are the functions ensuring compliance and boosting performance. The modular system suite includes components for Production Planning and Scheduling, Master Batch Record Management, Electronic Batch Recording, Weighing and Dispensing, Material Flow Control and Tracking, Equipment Integration, Warehouse Management, Quality Management, and Performance Management based on KPI Analysis. Werum’s solutions and services range from software consulting, creation of functional specifications, and software development to turn-key delivery of integrated and validated Manufacturing Execution Systems. Founded in 1969, the IT company employs more than 280 people in its headquarters in Lueneburg, Northern Germany, the regional offices in West and Southwest Germany, and the subsidiary in Parsippany, New Jersey, USA.
Werum Software & Systems, Tel. USA +1 (973) 644-4000, Tel. Europe +49 (0)4131 8900-0, Press Contact: Volker Mensing, mensing@werum.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Organon Counts on PAS-X for their Production of Active Pharmaceutical Ingredients here
News-ID: 14658 • Views: …
More Releases from Werum Software & Systems

13th PAS-X User Group Meeting in Germany
13th PAS-X User Group Meeting in Germany
Werum Manufacturing IT Conference with record attendance
Lueneburg, Germany, 26 October 2011 – The 13th international PAS-X User Group Meeting took place from 13 to 14 October 2011 in Lueneburg, Germany, with a record attendance. On the invitation of Werum Software & Systems AG, almost 200 pharmaceuticals experts from approximately 20 countries exchanged their thoughts and ideas on the use of the PAS-X production management…

Indian pharmaceutical major Dr. Reddy's chooses to implement Werum's MES
Dr. Reddy's Laboratories Ltd. deploys PAS-X at Hyderabad site
Lueneburg, Germany, August 20, 2010 – Dr. Reddy's Laboratories Ltd. has decided to introduce PAS-X, the Manufacturing Execution System (MES) by Werum Software & Systems, at its site in Bachupally, Hyderabad.
Dr. Reddy's plans to optimize the efficiency, quality, compliance and performance of its production processes with PAS-X. Werum is the leading supplier of MES systems for the pharmaceutical and biopharmaceutical industries…

MSD Packaging Lines run with PAS-X
Lueneburg, Germany, August 17, 2010 - MSD deployed PAS-X, the Manufacturing Execution System (MES) by Werum Software & Systems, to run its packaging line operations in Heist-op-den-Berg, Belgium in order to increase the compliance level during packaging operations and subsequently shorten lead times to improve delivery performance. Operations include fully automated blister lines and filling lines, semi-automated packaging lines and manual packaging processes. The project for introducing PAS-X was completed…
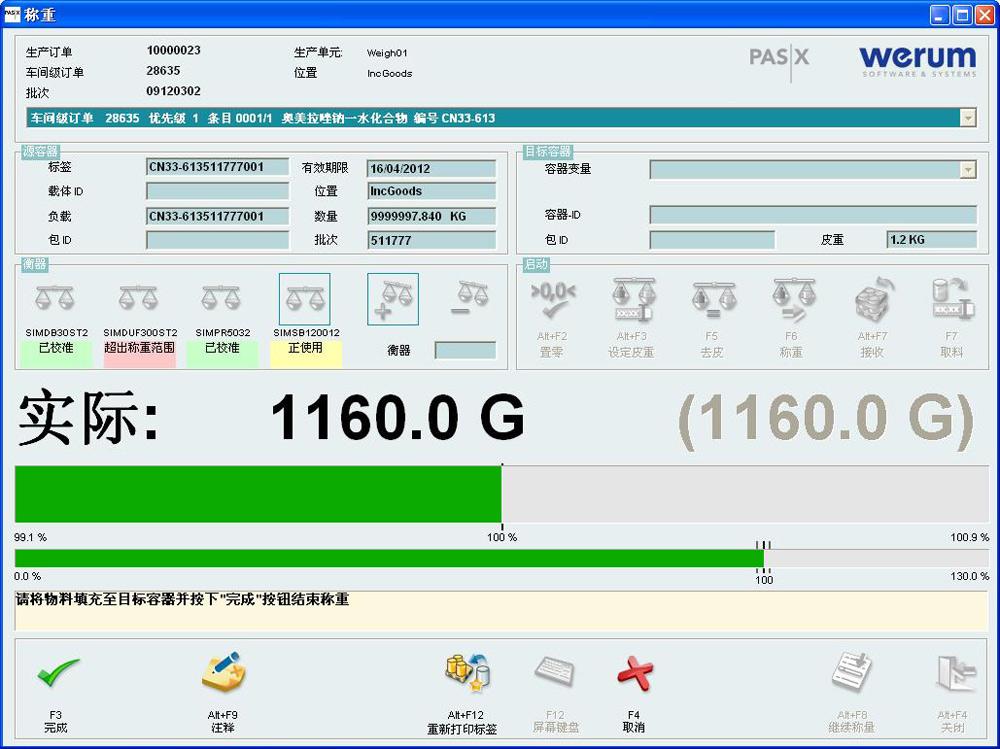
Successful rollout of PAS-X MES Weighing & Dispensing at AstraZeneca in India an …
Manufacturing Execution System projects completed successfully
Lueneburg, Germany, March 25, 2010 - Manufacturing Execution System (MES) software provider Werum Software & Systems has successfully finalized the installation of PAS-X MES in AstraZeneca's Wuxi site in the Jiangsu Province, China. This first-time MES project in the Chinese pharmaceutical industry follows an employment of Werum's PAS-X MES software in AstraZeneca's Indian production site in Bengaluru (formerly Bangalore) in 2009.
Both MES projects are part…
More Releases for Organon
Organon & Co. (NYSE: OGN) Investor Alert: Deadline in Lawsuit on July 22, 2025
A deadline is coming up on July 22, 2025 in the lawsuit filed for certain investors of Organon & Co. (NYSE: OGN) over alleged securities laws violations by Organon & Co.
Investors who purchased shares of Organon & Co. (NYSE: OGN) have certain options and there are strict and short deadlines running. Deadline: July 22, 2025. NYSE: OGN stockholders should contact the Shareholders Foundation at mail@shareholdersfoundation.com or call +1(858) 779…
Hypogonadism Pipeline | Clinical Trials, Key Companies -Mereo BioPharma, Merck, …
DelveInsight's, "Hypogonadism- Pipeline Insight, 2023" report provides comprehensive insights about 10+ companies and 10+ pipeline drugs in Hypogonadism pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Download the Sample Report to learn more
@https://www.delveinsight.com/sample-request/hypogonadism-pipeline-insight?utm_source=openpr&utm_medium=pressrelease&utm_campaign=dpr
Hypogonadism Overview
Hypogonadism occurs when your sex…
Muscle Relaxant Monitor Market | Blink Device, GE, IDMED, Organon Laboratories
The global muscle relaxant monitor market report is a comprehensive report that provides a detailed analysis of the current status and future trends of the muscle relaxant monitor market worldwide. This report provides valuable information to industry stakeholders by offering an in-depth perspective on market dynamics, competitive landscape, growth opportunities, and key challenges faced by industry participants.
From the perspective of market dynamics, this report explores the factors driving the growth…
Mirtazapine Tablets Market Analysis & Trends - Industry Forecast to 2027 |Novart …
LOS ANGELES, United States: The research study presented in this report offers complete and intelligent analysis of the competition, segmentation, dynamics, and geographical advancement of the global Mirtazapine Tablets market. It takes into account the CAGR, value, volume, revenue, production, consumption, sales, manufacturing cost, prices, and other key factors related to the global Mirtazapine Tablets market. The authors of the report have segmented the global Mirtazapine Tablets market as per…
Mirtazapine Tablets Market To Witness Huge Growth By 20277- Novartis, Mylan, Org …
Complete study of the global Mirtazapine Tablets market is carried out by the analysts in this report, taking into consideration key factors like drivers, challenges, recent trends, opportunities, advancements, and competitive landscape. This report offers a clear understanding of the present as well as future scenario of the global Mirtazapine Tablets industry. Research techniques like PESTLE and Porter's Five Forces analysis have been deployed by the researchers. They have also…
Ethinylestradiol Market Trend, Size, Share, Forecast 2021-2027 | Organon, Bayer, …
Ethinylestradiol market Research Report
LOS ANGELES, United States: QY Research offers an overarching research and analysis-based study on, “Global Ethinylestradiol Market Report, History and Forecast 2016-2027, Breakdown Data by Manufacturers, Key Regions, Types and Application“. This report offers an insightful take on the drivers and restraints present in the market. Ethinylestradiol data reports also provide a 5 year pre-historic and forecast for the sector and include data on socio-economic data…