Press release
Orphan Drugs Market Outlook to 2026 | Growth and Analysis by Top Vendors like Biogen Idec Limited, Eli Lilly and company, Bristol-Myers Squibb, Vertex pharmaceuticals, Bayer AG, Sanofi, Johnson & Johnson
Orphan disease, also known as a rare disease, affects a small percentage of the population. In some parts of the world, an orphan disease is a rare disease whose rarity means there's a lack of a market enormous enough to gain provision and assets for discovering treatments for that diseases, except by the government granting economically advantageous conditions to creating and selling such treatments.Orphan Drugs is medicine, vaccine, or in-vivo diagnostic mediator that is anticipated to diagnose, treat, and prevent an unusual disease or medical condition including chronic diseases. Most of the orphan diseases are genetic and thus are present through the individuals’ entire lifespan, and the indications may not immediately act. It is projected that approximately five to eight thousand rare diseases are present currently, and approximately 30 Million people are suffering from rare diseases in the European Union that is around 8% of total European population. Moreover, according to the National Organization for Rare Disorders (NORD), ratio of orphan disease occurrence is 1 in 10 people with more than 350 million people worldwide, and approximately 50% of the people affected by this disease are children.
Get PDF Sample Brochure: https://bit.ly/2zX5FyG
The symptoms of most of the orphan diseases may appear at birth or in childhood, for diseases such as cystic fibrosis, infantile spinal muscular atrophy, familial adenomatous polyposis (FAP), patent ductus arteriosus (PDA), and lysosomal storage disorder. However, some of the disease indications appear during middle age, this type of disease includes acute myeloid leukaemia, glioma, and renal cell carcinoma. The determined number of orphan diseases has notorious genetic origins. While, some diseases are the result of bacterial and viral infections due to degenerative causes.
Collaboration of Regulatory Organizations with Research Institutes to Propel the Global Market Growth
The Global Orphan Drugs market is majorly driven by growing number of people suffering orphan disease. Moreover, regulatory bodies are collaborating with research institutes and promoting research & development structure on the orphan disease, further propelling the growth in orphan drugs market. For instance, The European Union seventh frame work program for research and technical development is anticipated to bolster the research into these diseases. In addition, National Organization for Rare Disease (NORD) is authorized to provide the technical and scientific information for orphan diseases to patients, families, and public. These patient support programs offer information regarding disease-specific medication as well as treatment.
However, high initial investment and high treatment cost might hinder growth in global orphan drugs market. Moreover, the regulatory framework and standards vary from country to country, making it difficult for key players to operate on a worldwide level.
Global Orphan Drugs Market Taxonomy:
The global orphan drugs market is segmented on the basis of disease type, product type, distribution channel, and geography.
Key players in Global Orphan Drugs Market:
Hoffmann- La Roche
Celgene Corporation
Alexion Pharmaceuticals, Inc.
Novartis AG
Takeda Pharmaceuticals Company Limited
Biogen Idec Limited
Eli Lilly and company
Bristol-Myers Squibb
Vertex pharmaceuticals, Inc.
Bayer AG
Sanofi
Johnson & Johnson
Inquire For More Information: https://bit.ly/2B7vVs0
About Polaris Market Research:
Polaris Market Research is a global market research and consulting company. We strive to provide our customers with updated information on innovative technologies, high growth markets, emerging business environments and latest business-centric applications, thereby helping them always to make informed decisions and leverage new opportunities. The company specializes in providing exceptional market intelligence and in-depth business research services for our clientele spread across different enterprises.
Contact us:
Mr. Neel
Corporate Sales, USA
Polaris Market Research
Phone: 1-646-568-9980
Email: neel@polarismarketresearch.com
Web: https://www.polarismarketresearch.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Orphan Drugs Market Outlook to 2026 | Growth and Analysis by Top Vendors like Biogen Idec Limited, Eli Lilly and company, Bristol-Myers Squibb, Vertex pharmaceuticals, Bayer AG, Sanofi, Johnson & Johnson here
News-ID: 1373897 • Views: …
More Releases from Polaris Market Research & Consulting
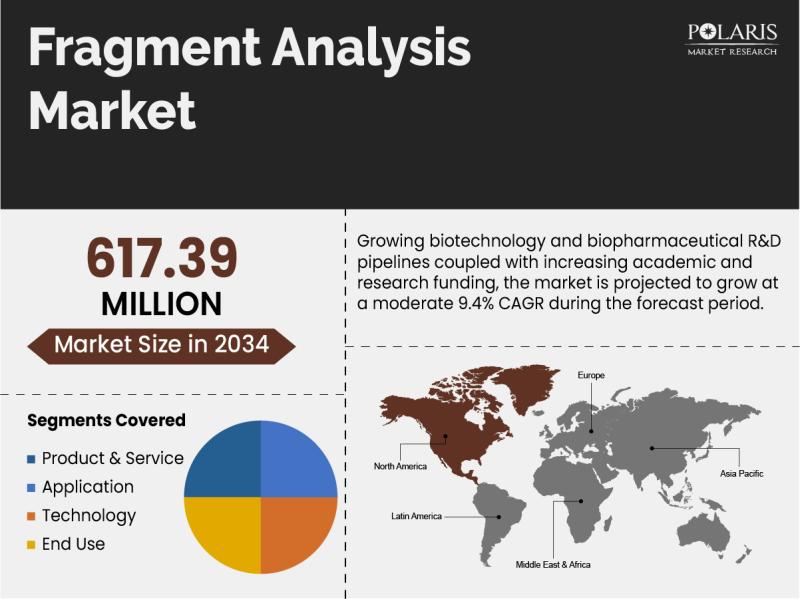
Fragment Analysis Market Size Projected to Reach USD 617.39 Million by 2034, Gro …
Global Fragment Analysis Market is currently valued at USD 275.72 Million in 2025 and is anticipated to generate an estimated revenue of USD 617.39 Million by 2034, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 9.4% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2026 - 2034.
Polaris Market Research recently introduced the latest update on Fragment Analysis Market…
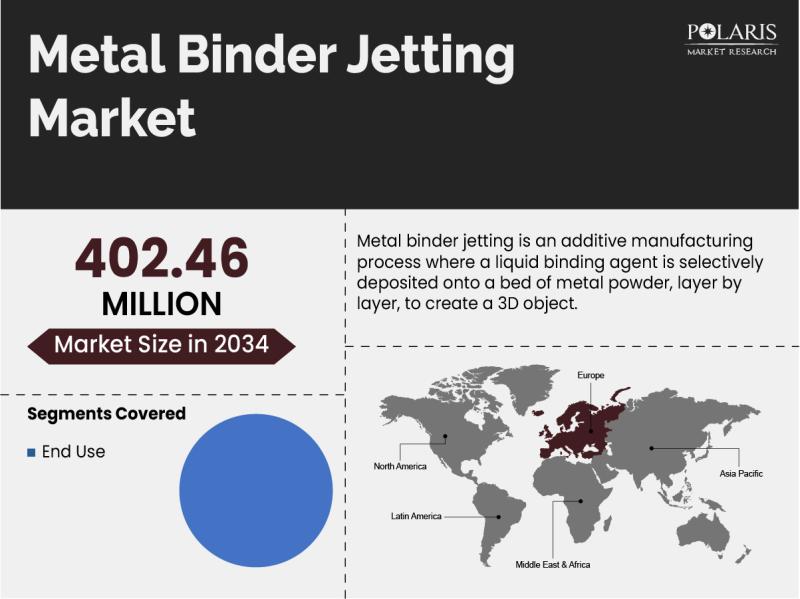
Metal Binder Jetting Market Growth Projected at 10.6% CAGR, Reaching USD 402.46 …
Global Metal Binder Jetting Market is currently valued at USD 147.51 Million in 2024 and is anticipated to generate an estimated revenue of USD 402.46 Million by 2034, according to the latest study by Polaris Market Research. Besides, the report notes that the market exhibits a robust 10.6% Compound Annual Growth Rate (CAGR) over the forecasted timeframe, 2025 - 2034.
Polaris Market Research recently introduced the latest update on Metal Binder…
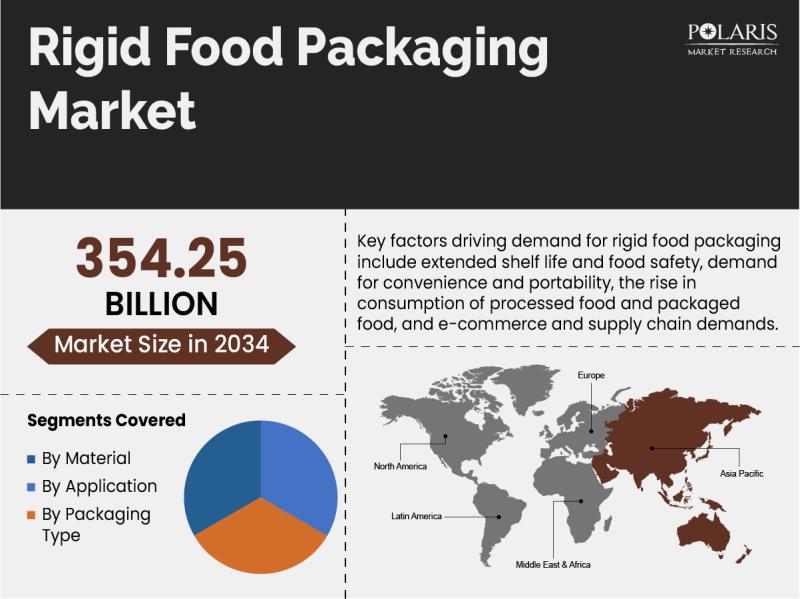
Rigid Food Packaging Market to Reach USD 354.25 Billion by 2034, Growing at a CA …
The quantitative market research report published by Polaris Market Research on Rigid Food Packaging Market aims to educate users with an in-depth understanding of a rapidly growing market. The study details important facts and figures, expert opinions, and major developments across the globe. The research study serves as a vital source of information with the historical data, Rigid Food Packaging Market size, financial data, and projected future growth. All the…
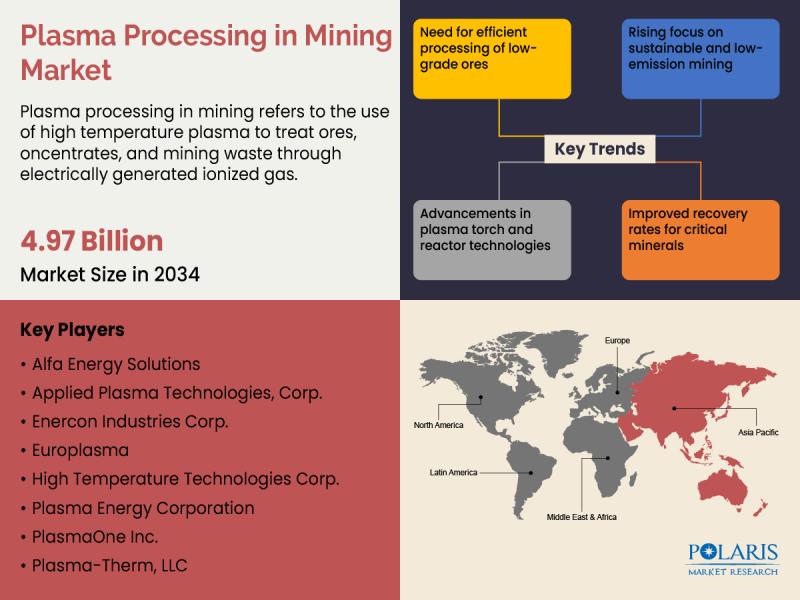
Global Plasma Processing in Mining Market to Reach USD 4.97 Billion by 2034, Reg …
Market Size and Share:
The global plasma processing in mining market is estimated to reach approximately USD 2.62 billion in 2025 and is expected to experience steady growth from 2026 to 2034, expanding at a projected CAGR of 7.4% during the forecast period.
Polaris Market Research has introduced the latest market research report titled Plasma Processing in Mining Market that highlights the major revenue stream for the forecast period. The report contains…
More Releases for Orphan
Acquired Orphan Blood Disease Market
Acquired Orphan Blood Disease Market to reach over USD 18.93 billion by the year 2031 - Exclusive Report by InsightAce Analytic
According to a new report by InsightAce Analytic, the "Acquired Orphan Blood Disease Market" in terms of revenue was estimated to be worth $8.65 billion in 2023 and is poised to reach $18.93 billion by 2031, growing at a CAGR of 10.47% from 2024 to 2031.
Get Free Access to…
Orphan Drugs Market Size to Hit $3199.3 Billion by 2028 | Orphan Drugs Industry …
Market Overview:
According to our experience research team, Orphan Drugs Market was valued at USD 112.36 Billion in 2021, and the global Orphan Drugs industry is projected to reach a value of USD 3199.3 Billion by 2028, at a CAGR of 7.4% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology, Energy &…
Orphan Drugs for Cancer Pipeline Analysis
A huge market opportunity is offered by small patient population which suffers from rare or orphan diseases. Among the category of new orphan drugs, Oncology account for the largest disease group in recent years. It has been observed that majority of the orphan drugs in the clinical stages are for rare cancer disease drugs, and are in the late stages of the pipeline. Some of the drugs are being developed…
US Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the pharmaceuticals industry.…
Europe Orphan Drugs Pipeline Analysis
“Europe Orphan Drugs Pipeline Analysis” by PNS Pharma gives comprehensive insight on the various drug profiles under Orphan Drugs status in Europe. Research report covers all the ongoing drug development in various phases. Each drug profiles include detailed information like: Originator, Owner, Collaborator, Technology Provider, Licensee, Development Phase, Development Indications, Mechanism of Action, Chemical Formula, Country of Development and detailed analysis on the development process. The information for particular drug…
Global Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US & Europe, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the…
