Press release
Biosimilars And Follow-On Biologics Market to Witness Comprehensive Growth by 2026
Biosimilars is defined as a type of biological product that is similar to another drug, which has already been licensed (approved) by the US FDA or European Medicines Agency. Due to their high degree of similarity with the biological reference product, they have no clinically evidenced and meaningful differences from the reference product in terms of quality, safety or efficacy. These drugs are also coined as follow-on biologics and are mostly derived from biological sources such as bacterium and yeast. The constitution of the biosimilar drugs can be either small molecules such as human insulin or erythropoietin, or complex molecules such as monoclonal antibodies. Biosimilars are increasing gaining prominence given the loss of exclusivity of big branded drugs. In Europe, biosimilars can be marketed through independent applicant following expiry of patent and market exclusivity periods of the reference product. A good example for this is Pfizer acquisition of Hospira, to gain access to the latter’s attractive biosimilars portfolio. Regulatory harmonization, naming and labelling, innovative licensure norms and route to market for the biosimilar drugs are issues expected to gain attention and traction from big drug makers in the forthcoming years.Biosimilars and Follow-on Biologics Market: Drivers and Restraints
Drivers for the biosimilars market include big brand name drugs losing patent extensions, cuts in healthcare costs across nations, forming of incentivized pricing policies by companies in order to access high growth pharmerging markets and good development in pharmacovigilance procedures across the globe. Other factors increasing the demand for biosimilar drugs include rising disease incidences across the globe and better access to healthcare for all nations.
Request to Sample of Report @ https://www.futuremarketinsights.com/reports/sample/rep-gb-1250
Restraints for the market include constraints in developing and registering biosimilar drugs and the complexity in the manufacturing processes. The drugs are difficult to verify and have to undergo complex regulations. Further, the drugs are different from each other due to variability in raw material and in the manufacturing process, which is expected to deter the future development of biosimilar drugs. There are also risks in having an increase in the versions of an identical biosimilar drug.
Biosimilars and Follow-on Biologics Market: Segmentation
Biosimilars and Follow-on Biologics Market can be segmented into these following ways:
Segmentation by product class
epoetins;
filgrastims;
insulins;
growth hormones;
alfa interferons;
monoclonal antibodies;
beta interferons;
follitropins;
low-molecular-weight heparins (LMWH).
Segmentation by application
Rheumatoid arthritis
Anemia
Cancer
Diabetes
Others
Segmentation by regions
Report URL @ https://www.futuremarketinsights.com/reports/biosimilars-and-follow-on-biologics-market
Biosimilars and Follow-on Biologics Market: Overview
Biosimilar market is expected to gain prominence over the forthcoming years due to leading biologic drugs expected to lose exclusivity over the next seven years. Further, biosimilar drugs, once formed, are expected to generate cost savings for the patient population. Competition is expected to be limited in the market as the drugs are expected to be formed using various types of innovative technologies. Biosimilars may generate smaller savings for drug makers because of their complexity as well as regulatory challenges of getting FDA approvals.
Biosimilars and Follow-on Biologics Market: Region-wise Outlook
Depending on geographic regions, biosimilar drug market is segmented into seven key regions: North America, South America, Eastern Europe, Western Europe, Asia Pacific, Japan, and Middle East & Africa.
In terms of geography, Europe dominates the market, followed by Asia-Pacific. However, rising technological advancement in healthcare and systematic drug review process will drive the markets over North America, Japan and other regions. Europe dominates the biosimilars market driven by technically advanced healthcare infrastructure and high patient awareness & regulatory harmonization. Increasing funding for development of biosimilar drugs, availability of high-quality research infrastructure and strategies developed by drug makers to restrict entry of new players. Emerging markets include Eastern European countries followed by countries in Eastern Africa. Rising disease incidences in these countries is expected to prove favorable for the growth of the biosimilar drug market.
Request Report for TOC @ https://www.futuremarketinsights.com/toc/rep-gb-1250
Biosimilars and Follow-on Biologics Market: Key Players
Some of the key players in biosimilar market are Pfizer Inc. (AC. Hospira), Sandoz International GmbH, , Teva Pharmaceutical Industries Ltd., Dr. Reddy’s Laboratories, Biocon Limited, Mylan, Inc. , Amgen, Celltrion Inc., Roche Diagnostics, and Merck KGaA.
About Us
Future Market Insights is the premier provider of market intelligence and consulting services, serving clients in over 150 countries. FMI is headquartered in London, the global financial capital, and has delivery centres in the U.S. and India.
FMI’s research and consulting services help businesses around the globe navigate the challenges in a rapidly evolving marketplace with confidence and clarity. Our customised and syndicated market research reports deliver actionable insights that drive sustainable growth. We continuously track emerging trends and events in a broad range of end industries to ensure our clients prepare for the evolving needs of their consumers.
U.S. Office
616 Corporate Way, Suite 2-9018,
Valley Cottage, NY 10989,
United States
T: +1-347-918-3531
F: +1-845-579-5705
Email: sales@futuremarketinsights.com
Web: https://www.futuremarketinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars And Follow-On Biologics Market to Witness Comprehensive Growth by 2026 here
News-ID: 1336429 • Views: …
More Releases from Future Market Insights
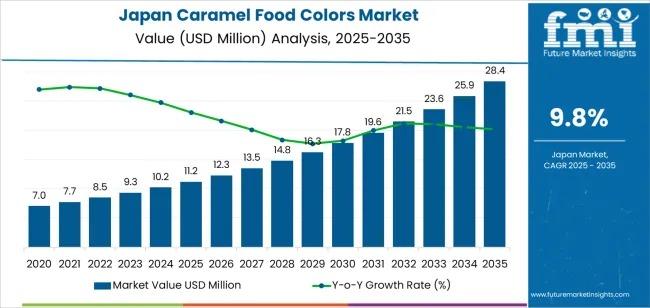
Japan Caramel Food Colors Industry Outlook to 2036: Strategic Insights for R&D, …
The Japanese caramel food colors market is on a steady growth trajectory, with demand projected to rise from USD 11.2 million in 2025 to USD 28.4 million by 2035, registering a CAGR of 9.8%. The initial phase of the forecast period (2025-2030) anticipates a steady increase in demand, reaching approximately USD 17.8 million by 2030, driven by the expanding use of caramel colors across confectionery, dairy, and baked goods.
The market's…
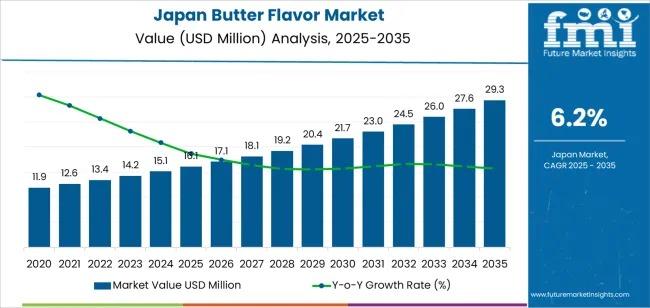
Comprehensive Analysis of the Japan Butter Flavor Market: Technology Evolution, …
The demand for butter flavor in Japan is projected to rise from USD 16.1 million in 2025 to USD 29.4 million by 2035, reflecting a steady compound annual growth rate (CAGR) of 6.2%. This growth is underpinned by increasing adoption across bakery products, confectionery items, and dairy-based preparations, as manufacturers seek to enhance taste experiences and deliver authentic dairy character in a wide range of food offerings.
The Japanese bakery and…
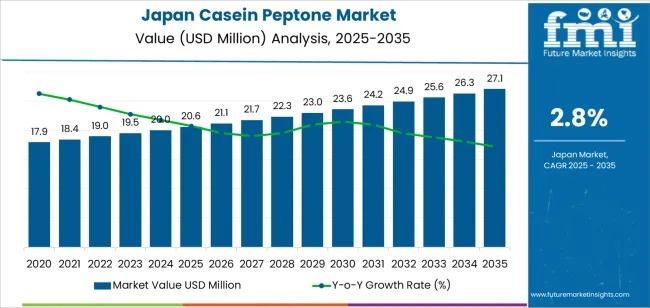
Japan Casein Peptone Market Deep-Dive 2026-2036: Strategic Forecasts, Market Ent …
The demand for casein peptone in Japan is projected to grow steadily, reaching USD 27.1 million by 2035, up from USD 20.6 million in 2025, reflecting a compound annual growth rate (CAGR) of 2.8%. During the early forecast period (2025-2030), demand is expected to rise from USD 20.6 million to approximately USD 23.6 million, supported by its widespread applications in biotechnology, pharmaceuticals, and food industries. Casein peptone continues to play…

Global Boride Powder Market Size, Share & Forecast: High-Growth Segments, Value …
The global boride powder market is valued at USD 19.7 billion in 2025 and is projected to reach USD 32.2 billion by 2035, advancing at a steady 5.0% CAGR over the forecast period. This upward trajectory reflects increasing adoption of boride-based compounds in aerospace technology, high-temperature processing environments, and advanced coating applications, where exceptional thermal stability, corrosion resistance, and mechanical strength are essential for operational performance and product reliability.
Key Market…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
