Press release
Infliximab Market - Analysis and Forecast 2018 - 2026 Explored in Latest Research
Biosimilars are biological products that are similar to FDA-approved drug known as branded drug. Biosimilars are drugs licensed by the European Medicines Agency (EMA) and the U.S. FDA. These do not have significant differences from the reference products in terms of purity, effectiveness, and safety. Biosimilar drugs can only be permitted for conditions and indications that have been formerly accepted for the reference product by government regulatory agencies and organizations, when the original products patents expires. Compared to generic drugs, biosimilars show more molecular complexity. These are also quite sensitive to modifications in the manufacturing process.Infliximab (Remicade) is a monoclonal antibody first manufactured by Janssen Biotech, Inc. in collaboration with Merck & Co. The U.S. Food and Drug Administration (FDA) first approved it in 1998. It is used in the treatment of active ulcerative colitis, rheumatoid arthritis, active and spinal psoriatic arthritis, Crohn’s disease (inflammatory bowel disease) in both pediatric and adult patients, and plaque psoriasis. Infliximab binds to chemical messenger tumor necrosis factor (TNF-α). It is one of the important part of autoimmune reaction. TNF-α stimulates the overall messenger cascade. Infliximab seems to act by binding to the receptor in the cell.
Obtain Report Details @ https://www.transparencymarketresearch.com/infliximab-market.html
The global infliximab market is projected to grow at a rapid pace in the next few years. Increase in incidence of hereditary and autoimmune diseases such as plaque psoriasis and rheumatoid arthritis drive the global market. The number of people suffering from Crohn’s disease and ulcerative colitis (UC) has increased in various regions in the past few years. All the inflammatory bowel diseases (IBD) are associated with considerable morbidity cases with substantial use of health care resources. Early patent expiry, discounted pricing on branded drugs, and quick response time due to intravenous mode of administration propel the market.
Additionally, entry of biosimilars into the market has the ability to provide significant financial relief to the current health care systems. However, side effects of the drug treatment such as histoplasmosis (fungal infection), tuberculosis (TB), bacterial sepsis, and others restrain the global infliximab market. Hence, manufacturers of the drug have been mandated to include warnings to alert both patients and health care professionals. Additionally, lack of FDA-approved facilities, especially in developing countries, to manufacture the drug are the factors expected to restrain the market.
Download Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=43640
The global infliximab market can be segmented based on product and application. In terms of product, the global infliximab market can be bifurcated into biologics and biosimilars. Based on application, the global infliximab market can be divided into rheumatoid arthritis, ankylosing spondylitis, plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis.
Geographically, the global infliximab market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America and Europe are projected to dominate the market, followed by Asia Pacific and Latin America. Dominance of North America and Europe is attributed to launch of biosimilars after patent expiry.
The market in Asia Pacific is anticipated to grow at a rapid pace in the next few years. This is due to presence of rapidly developing economies such as China and India, increase in awareness about health and hygiene, enhanced health care infrastructure, and unexploited market as increase in geriatric population that lacks adequate diagnostic & treatment solutions. Additionally, rise in government support for the manufacture of biosimilars drugs and increase in awareness and adoption of biosimilars is anticipated to drive the global infliximab market.
Enquiry for Discount on this Report @ https://www.transparencymarketresearch.com/sample/sample.php?flag=D&rep_id=43640
Key players in the global infliximab market are Merck & Co., Celltrion, Inc., Alvogen, Janssen Biotech, Inc., NAPP Pharmaceuticals, Nippon Kayaku, and Pfizer, Inc. (AC. Hospira), among others.
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Contact Us
Transparency Market Research
State Tower,
90 State Street, Suite 700
Albany, NY 12207
United States
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: http://www.transparencymarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Infliximab Market - Analysis and Forecast 2018 - 2026 Explored in Latest Research here
News-ID: 1077352 • Views: …
More Releases from Transparency Market Research

Wireless Sensors Market to be Worth USD 80.6 Bn by 2036 - By Sensor Type / By Co …
The global wireless sensors market is witnessing strong growth momentum, driven by rising digital transformation across industrial, infrastructure, healthcare, and smart city ecosystems. According to industry analysis, the wireless sensors market was valued at US$ 17.4 billion in 2025 and is projected to reach US$ 80.6 billion by 2036, expanding at a robust CAGR of 14.7% during the forecast period from 2026 to 2036.
Access an overview of significant conclusions from…
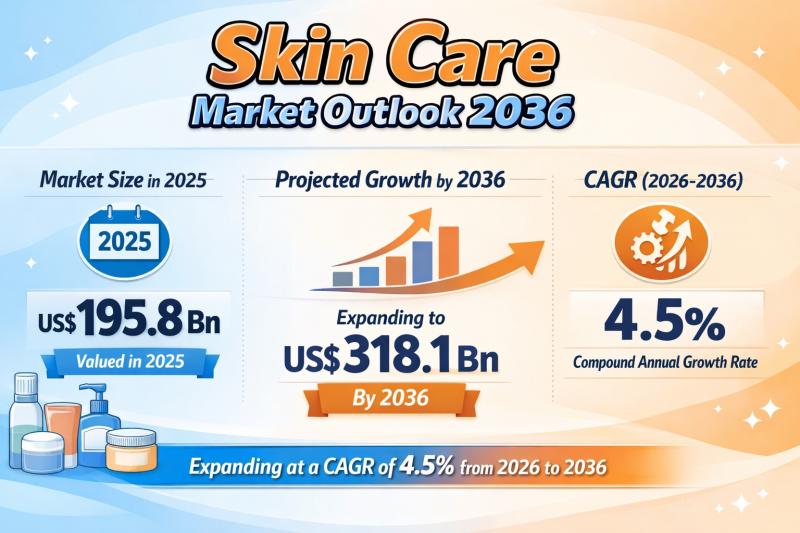
Skin Care Market to be Worth USD 318.1 Bn by 2036 - By Type / By Skin Type / By …
The global skin care market continues to demonstrate steady growth, reflecting the increasing integration of skincare into daily health and wellness routines. The market was valued at US$ 195.8 billion in 2025 and is projected to reach US$ 318.1 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.5% from 2026 to 2036.
Review critical insights and findings from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=340
The industry growth is…
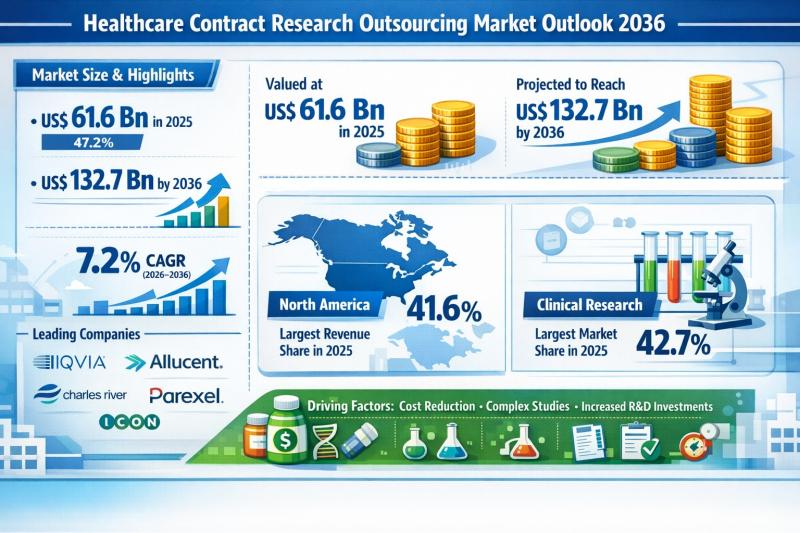
Healthcare Contract Research Outsourcing Market Outlook 2036: Market to Reach US …
The global Healthcare Contract Research Outsourcing Market was valued at US$ 61.6 Bn in 2025 and is projected to reach US$ 132.7 Bn by 2036, expanding at a compound annual growth rate (CAGR) of 7.2% from 2026 to 2036. The market's steady expansion reflects the growing reliance of pharmaceutical, biotechnology, and medical device companies on Contract Research Organizations (CROs) to optimize development costs and accelerate time-to-market.
In 2025, North America accounted…
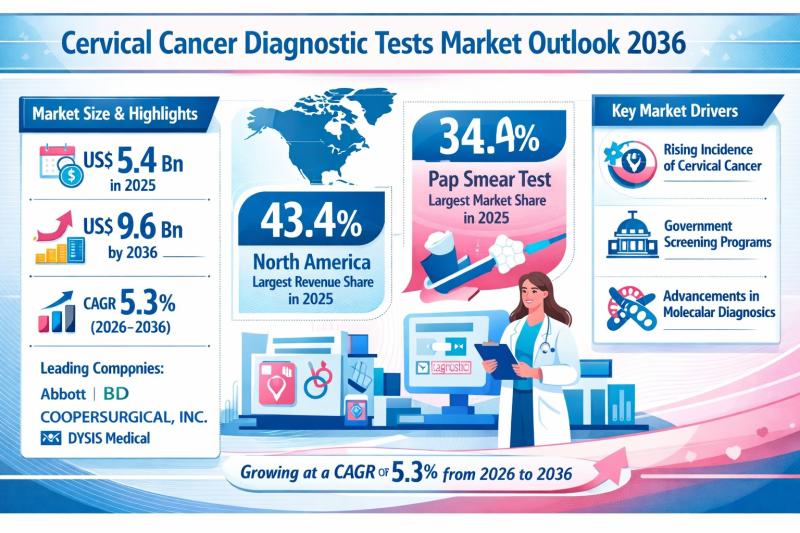
Cervical Cancer Diagnostic Tests Market Outlook 2036: Global Industry to Reach U …
The global cervical cancer diagnostic tests market is on a steady growth trajectory, reflecting the rising global focus on women's health, preventive oncology, and early disease detection. Valued at US$ 5.4 Bn in 2025, the market is projected to reach US$ 9.6 Bn by 2036, expanding at a compound annual growth rate (CAGR) of 5.3% from 2026 to 2036.
Cervical cancer remains one of the most common cancers affecting women worldwide,…
More Releases for Infliximab
Adalimumab, Infliximab, and Etanercept Biosimilars Market Trends 2024
"The Business Research Company recently released a comprehensive report on the Global Adalimumab, Infliximab and Etanercept Biosimilars Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your…
Infliximab Biosimilar Market Research Report 2024
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/1060
According to the latest research by InsightAce Analytic, the global…
Infliximab Biosimilar Insight, 2023 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2023" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
Infliximab Biosimilar Insight, 2023 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2023" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
Infliximab and Biosimilar Market 2021 | Detailed Report
Infliximab and Biosimilar Market Forecasts report provided to identify significant trends, drivers, influence factors in global and regions, agreements, new product launches and acquisitions, Analysis, market drivers, opportunities and challenges, risks in the market, cost and forecasts to 2027.
Get Free Sample PDF (including full TOC, Tables and Figures) of Infliximab and Biosimilar Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=5048518
The report provides a comprehensive analysis of company profiles listed below:
- Janssen Biotech
- Merck and…
