Press release
Biosimilars Insulin Market: Growth Analysis by 2016 - 2024
Biosimilars is a biological product designed to have similar active properties to the one that has been previously licensed and has no clinically significant differences in terms of safety and effectiveness. Biosimilars insulin is a type of biosimilars where it is indistinguishable to the reference insulin product and is already been approved by FDA or licensed. The producers of biosimilars use the similar manufacturing techniques as of the patented product but not likely identical to that used by the patent holder.Due to increasing insulin manufacturers from the developed and developing countries, the patents for insulin formulations had neared expiry or were ended. This created a necessity to seek approvals on biosimilar insulin for the not yet established companies in the highly regulated markets such as Europe and United States. In 2014, The European Commission (EC) first granted insulin treatment through the biosimilars pathway to Eli Lilly and Company and Boehringer Ingelheim GmbH.
Request to Sample Report @ https://www.persistencemarketresearch.com/samples/11674
Lilly/Boehringer Ingelheim developed a biosimilar insulin called “Insulin glargine” and was the fourth diabetes product which was approved from Lilly-Boehringer Ingelheim Alliance in Europe. Basaglar is the first “biosimilar” insulin product to be approved and launched in U.S. which was developed by the alliance of Lilly/Boehringer Ingelheim.
Biosimilar insulin proved to have an effective treatment for patients suffering from diabetes mellitus by reducing the cost for the treatment. There are some companies who have developed devices which allow the safe and less painful delivery of biosimilar insulin thus making the device a market differentiator even though it’s chemically equivalent to the branded product. Many companies in the emerging economies have started developing biosimilar insulin due to the favorable regulatory requirements as compared to the companies in developed countries where their stringent regulatory requirements. As the regulatory requirements for the approval process of biosimilars are considerably higher than those required for the generic drugs and entail the comprehensive analysis for each of its characteristics.
Biosimilars have increased the opportunities in healthcare services and have decreased the expenditures creating a significant competition between the companies manufacturing branded products and biosimilars. Globally, the major factors responsible for surging the demand for biosimilars insulin in the market are increase in diagnosed and undiagnosed diabetes, patent expiry for insulin, growing population. Diabetes is considered to be one of the fastest growing disease and attribute to 3 million deaths annually, and has become a pandemic. Increasing poverty level in developing countries has created a pressure on the healthcare industry to develop cost effective products and treatment options especially for diabetes patients. The only aspects which can affect the biosimilars market are safety and efficacy of new biosimilars also the stringent regulatory bodies leading to delayed approvals.
According to the Biosimilars Pipeline Database, there are total 1,357 product entries for biobetters and biosimilars constitute 737 of them (March, 2016). Among the top targeted reference products, insulin and analogs constitute 51 and the companies producing it include Biocon, Mylan N.V., Sanofi, Novo Nordisk and others. Sanofi, a multinational pharmaceutical company is also developing LixiLan, a mix of basal insulin with GLP-1 which is directed to treat for type 2 diabetes. Eli Lilly and Company, a U.S. based company is currently running Phase III clinical trials for a non-insulin drug Empagliflozin (BI10773) targeting the treatment for type 1 diabetes.
Request Report for TOC @ https://www.persistencemarketresearch.com/toc/11674
The new trend in the market is developing ultra-rapid acting insulins to replace Humalog and Novolog (patents expired) which is important for pump users and disorder related to real-time artificial pancreas. There are new attempts made by companies to create oral insulin such as ingestible capsules for better drug delivery. Most of the biosimilars have reached Phase III and are soon to get approved by the respective regulatory bodies and will reach the market with cheaper prices as compared to the branded products. Various multinationals are forming alliances and partnerships to develop biosimilar insulin such as Eli Lilly and Company and Boehringer Ingelheim Gmb, Biocon Limited and Pfizer Inc., Mylan N.V. and Momenta Pharmaceuticals.
About Us
Persistence Market Research (PMR) is a third-platform research firm. Our research model is a unique collaboration of data analytics and market research methodology to help businesses achieve optimal performance.To support companies in overcoming complex business challenges, we follow a multi-disciplinary approach. At PMR, we unite various data streams from multi-dimensional sources. By deploying real-time data collection, big data, and customer experience analytics, we deliver business intelligence for organizations of all sizes.
Contact Us
Persistence Market Research
305 Broadway
7th Floor, New York City,
NY 10007, United States,
USA – Canada Toll Free: 800-961-0353
Email: sales@persistencemarketresearch.com
Web: http://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilars Insulin Market: Growth Analysis by 2016 - 2024 here
News-ID: 1071452 • Views: …
More Releases from Persistence Market Research Pvt. Ltd

Innovative Rehabilitation Technologies Drive Growth in Stroke Rehabilitation Mar …
Stroke Rehabilitation Market Size
The global stroke rehabilitation market size was valued at US$ 266.4 million in 2021 and is expected to grow at a CAGR of 9% from 2022 to 2032. Revenue generated from the sales of stroke rehabilitation solutions will surge to US$ 679.6 Million by the end of 2032.
The growth of the market is being driven by the increasing incidence of strokes, the growing demand for innovative rehabilitation…
Launch of New Laser-Based Devices by Key Players Fueling Growth of Global Aesthe …
Market Study on Aesthetic Lasers
The aesthetic lasers market reached US$2.8 billion in global revenue in 2021, and it will continue to expand quickly, with a value CAGR of 6.4%, to reach US$5.5 billion by the end of 2032. The growth of the market is being driven by the increasing demand for minimally invasive cosmetic procedures, rising disposable income, technological advancements, and growing medical tourism.
Stay Ahead with Market Intelligence Get Free…

Predictive Insights for Precision Medicine: The Global Biosimulation Market is S …
Biosimulation Market Research
The Global Biosimulation Market Size was valued at USD 2.9 billion in 2022 and is expected to reach USD 10.0 billion by 2030, registering a CAGR of 16.9% during the forecast period. The growth of the biosimulation market is being driven by the increasing use of biosimulation in a variety of applications, such as drug discovery, clinical trials, and toxicity prediction.
Market Overview
The biosimulation market has experienced rapid growth…
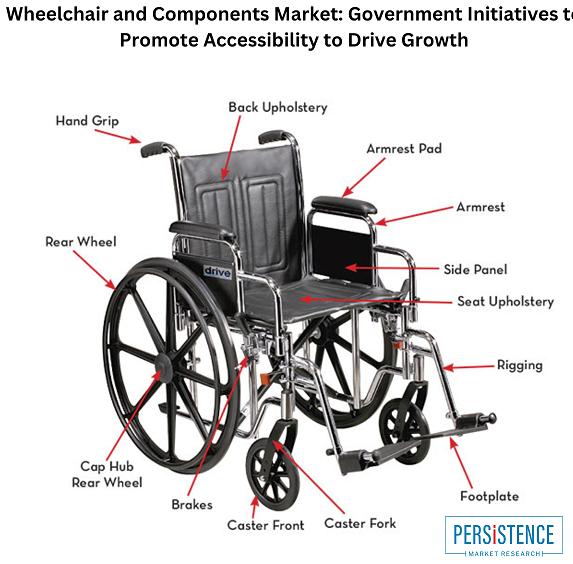
Wheelchair and Components Market: Growing Demand for Lightweight and Smart Wheel …
Wheelchair and Components Market
The global wheelchair and components market is projected to reach USD 17.9 billion by 2033, at a CAGR of 3.7% from 2022-2033. The growth of the market is driven by the increasing prevalence of disabilities, the rising geriatric population, and technological advancements in wheelchair design and manufacturing.
Click Here to Get Free Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/12961
The global wheelchair and components market has witnessed significant growth in…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
