Press release
Healthcare Regulatory Affairs Outsourcing Market market value of over US$ 700 Mn by the end of 2025
Global Healthcare Regulatory Affairs Outsourcing Market: OverviewConsistent evaluation of cost-saving options such as regulatory affairs outsourcing is carried out by several drug/device manufacturers in order to streamline their operations and ensure product safety and maintain public healthcare.
Request For Sample @https://www.futuremarketinsights.com/reports/sample/rep-gb-6355
The global health care regulatory affairs outsourcing market includes services like regulatory writing and publishing, clinical trial applications, etc. A new research report by Future Market Insights provides an in-depth analysis of the global regulatory affairs outsourcing market. This comprehensive research report is titled ‘Healthcare Regulatory Affairs Outsourcing Market: Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2017 – 2025’. It includes all the crucial numbers of the market along with the dynamics impacting it. According to this research report the global healthcare regulatory affairs outsourcing market is expected to reach a market revenue of over US$ 2,000 Mn by the end of 2025, growing at a stellar CAGR of 12.2% over the forecast period. The growth of the market is triggered largely by the increasing investments in the healthcare industry along with the increase in research and developments taking place. Also increasing focus on CMOs and CROs has been observed in the market. The reports also depicts that Asia Pacific is expected to lead the global healthcare regulatory affairs outsourcing market during 2017-2025. Low cost, fast turnaround time and easy availability of the skilled and trained professionals will further result in increased outsourcing in APAC countries.
Global Healthcare Regulatory Affairs Outsourcing Market: Segmental Analysis
Based on End Users, mid-size pharmaceutical companies leads the market with a high market size expected during the forecast period, growing at a CAGR of 13.8% during the forecast period. However Biotechnology companies are also progressing at a high rate and is expected to give a strong competition to mid-size pharma companies in terms of growth rate expected during the forecast period.
On the basis of Services, regulatory writing and publishing service segment is expected to dominate the market with a market share of over US$ 800 Mn by the end of 2025. However the segment is much behind the other services in terms of growth rate. Regulatory consulting and legal representation is the service which is expected to register fastest growth at a CAGR of 14.3% during the forecast period.
In terms of Region, Asia Pacific is expected to be the largest region with a high market valuation. However, North America is also expected to give tough competition to APAC. Both these regions are expected to reflect a valuation higher than US$ 720 Mn by 2025 end. However North America lags behind in the race with comparatively less CAGR expected during the forecast period. Whereas, Asia Pacific is expected to register the fastest growth rate of 13.2% CAGR during the forecast period.
Request For TOC @ https://www.futuremarketinsights.com/askus/rep-gb-6355
Global Healthcare Regulatory Affairs Outsourcing Market: Competitive Landscape
The research report consists of a brief profile of all the major players leading in the industry. It also includes a SWOT analysis of these companies. The leading players mentioned in the report includes companies like Clinilabs, Inc., Accell Clinical Research, LLC., Freyr Solutions, The Weinberg Group Inc., Covance, Inc., (LabCorp), Pharmaceutical Product Development LLC, ICON plc., Sciformix Corporation etc.
ABOUT US:
Future Market Insights (FMI) is a leading market intelligence and consulting firm. We deliver syndicated research reports, custom research reports and consulting services, which are personalized in nature.
FMI delivers a complete packaged solution, which combines current market intelligence, statistical anecdotes, technology inputs, valuable growth insights, an aerial view of the competitive framework, and future market trends.
CONTACT:
Future Market Insights
616 Corporate Way, Suite 2-9018,
Valley Cottage, NY 10989,
United States
T: +1-347-918-3531
F: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: http://www.futuremarketinsights.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Healthcare Regulatory Affairs Outsourcing Market market value of over US$ 700 Mn by the end of 2025 here
News-ID: 1069798 • Views: …
More Releases from Future Market Insights
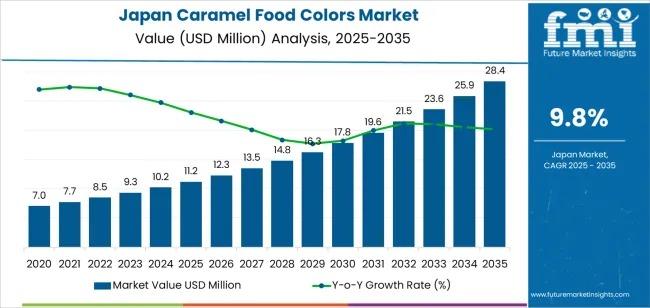
Japan Caramel Food Colors Industry Outlook to 2036: Strategic Insights for R&D, …
The Japanese caramel food colors market is on a steady growth trajectory, with demand projected to rise from USD 11.2 million in 2025 to USD 28.4 million by 2035, registering a CAGR of 9.8%. The initial phase of the forecast period (2025-2030) anticipates a steady increase in demand, reaching approximately USD 17.8 million by 2030, driven by the expanding use of caramel colors across confectionery, dairy, and baked goods.
The market's…
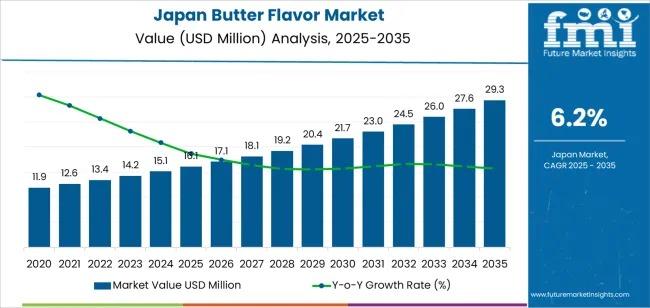
Comprehensive Analysis of the Japan Butter Flavor Market: Technology Evolution, …
The demand for butter flavor in Japan is projected to rise from USD 16.1 million in 2025 to USD 29.4 million by 2035, reflecting a steady compound annual growth rate (CAGR) of 6.2%. This growth is underpinned by increasing adoption across bakery products, confectionery items, and dairy-based preparations, as manufacturers seek to enhance taste experiences and deliver authentic dairy character in a wide range of food offerings.
The Japanese bakery and…
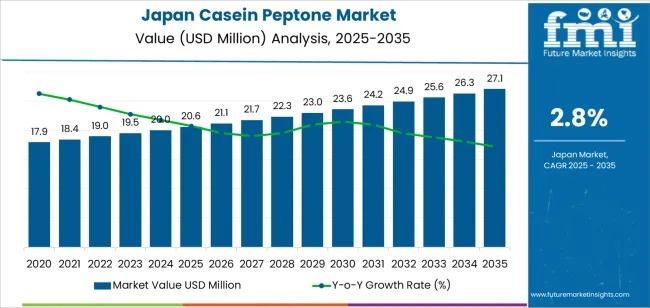
Japan Casein Peptone Market Deep-Dive 2026-2036: Strategic Forecasts, Market Ent …
The demand for casein peptone in Japan is projected to grow steadily, reaching USD 27.1 million by 2035, up from USD 20.6 million in 2025, reflecting a compound annual growth rate (CAGR) of 2.8%. During the early forecast period (2025-2030), demand is expected to rise from USD 20.6 million to approximately USD 23.6 million, supported by its widespread applications in biotechnology, pharmaceuticals, and food industries. Casein peptone continues to play…

Global Boride Powder Market Size, Share & Forecast: High-Growth Segments, Value …
The global boride powder market is valued at USD 19.7 billion in 2025 and is projected to reach USD 32.2 billion by 2035, advancing at a steady 5.0% CAGR over the forecast period. This upward trajectory reflects increasing adoption of boride-based compounds in aerospace technology, high-temperature processing environments, and advanced coating applications, where exceptional thermal stability, corrosion resistance, and mechanical strength are essential for operational performance and product reliability.
Key Market…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
